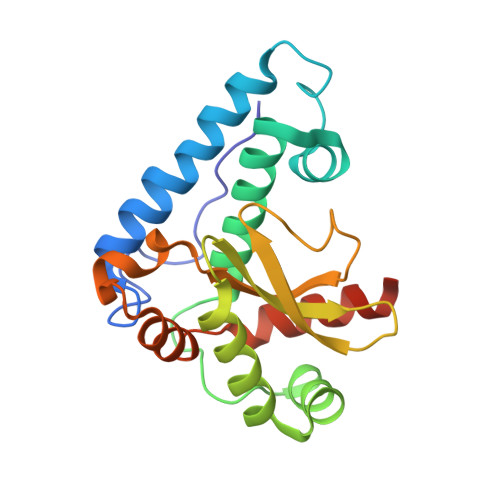
The Crystal Structure of Superoxide Dismutase from Plasmodium Falciparum.
Boucher, I.W., Brzozowski, A.M., Brannigan, J.A., Schnick, C., Smith, D.J., Kyes, S.A., Wilkinson, A.J.(2006) BMC Struct Biol 6: 20
- PubMed: 17020617
- DOI: https://doi.org/10.1186/1472-6807-6-20
- Primary Citation of Related Structures:
2BPI - PubMed Abstract:
Superoxide dismutases (SODs) are important enzymes in defence against oxidative stress. In Plasmodium falciparum, they may be expected to have special significance since part of the parasite life cycle is spent in red blood cells where the formation of reactive oxygen species is likely to be promoted by the products of haemoglobin breakdown. Thus, inhibitors of P. falciparum SODs have potential as anti-malarial compounds. As a step towards their development we have determined the crystal structure of the parasite's cytosolic iron superoxide dismutase.
Organizational Affiliation:
Structural Biology Laboratory, Department of Chemistry, University of York, York YO10 5YW, UK. boucher@ysbl.york.ac.uk