Crystal Structure of the Pb1 Domain of Nbr1
Mueller, S., Kursula, I., Zou, P., Wilmanns, M.(2006) FEBS Lett 580: 341
- PubMed: 16376336
- DOI: https://doi.org/10.1016/j.febslet.2005.12.021
- Primary Citation of Related Structures:
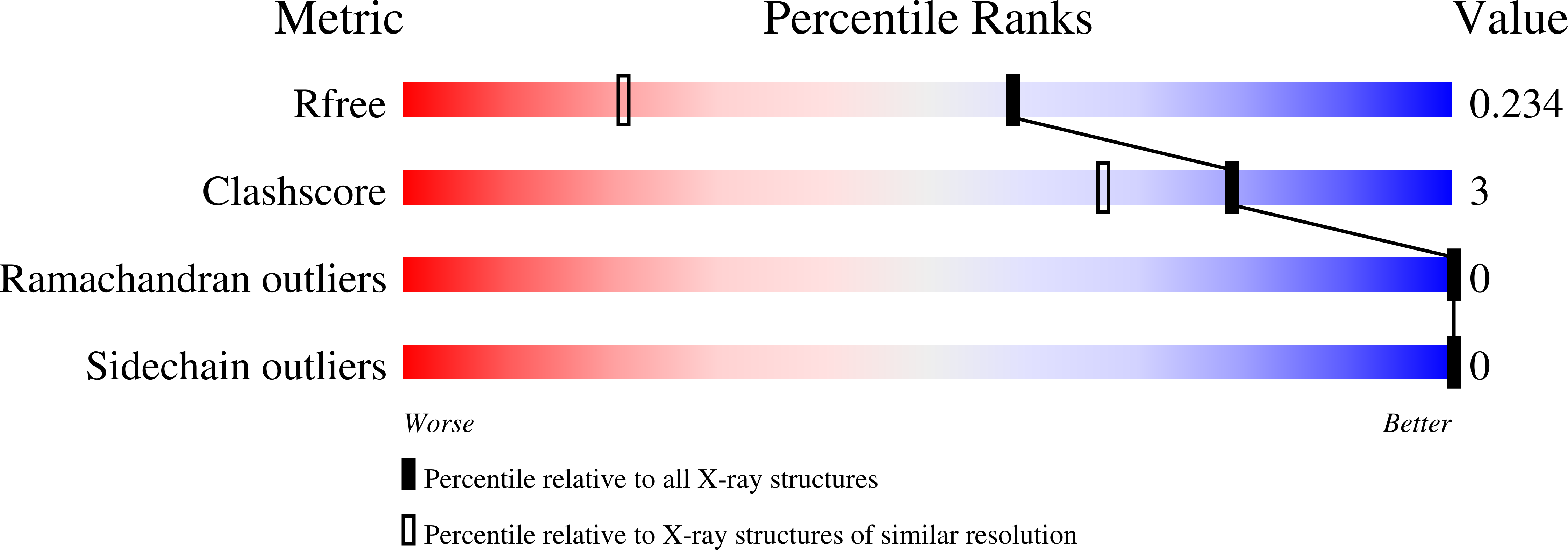
2BKF - PubMed Abstract:
The scaffold protein NBR1 is involved in signal transmission downstream of the serine/protein kinase from the giant muscle protein titin. Its N-terminal Phox and Bem1p (PB1) domain plays a critical role in mediating protein-protein interactions with both titin kinase and with another scaffold protein, p62. We have determined the crystal structure of the PB1 domain of NBR1 at 1.55A resolution. It reveals a type-A PB1 domain with two negatively charged residue clusters. We provide a structural perspective on the involvement of NBR1 in the titin kinase signalling pathway.
Organizational Affiliation:
EMBL-Hamburg Outstation, c/o DESY, Notkestrasse 85, D-22603 Hamburg, Germany.