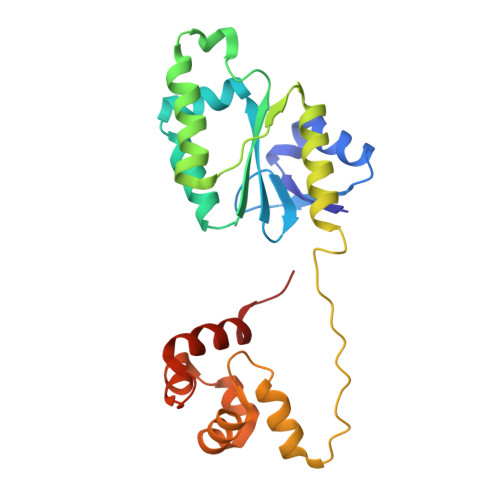
Structure of an XPF endonuclease with and without DNA suggests a model for substrate recognition.
Newman, M., Murray-Rust, J., Lally, J., Rudolf, J., Fadden, A., Knowles, P.P., White, M.F., McDonald, N.Q.(2005) EMBO J 24: 895-905
- PubMed: 15719018
- DOI: https://doi.org/10.1038/sj.emboj.7600581
- Primary Citation of Related Structures:
2BGW, 2BHN - PubMed Abstract:
The XPF/Mus81 structure-specific endonucleases cleave double-stranded DNA (dsDNA) within asymmetric branched DNA substrates and play an essential role in nucleotide excision repair, recombination and genome integrity. We report the structure of an archaeal XPF homodimer alone and bound to dsDNA. Superposition of these structures reveals a large domain movement upon binding DNA, indicating how the (HhH)(2) domain and the nuclease domain are coupled to allow the recognition of double-stranded/single-stranded DNA junctions. We identify two nonequivalent DNA-binding sites and propose a model in which XPF distorts the 3' flap substrate in order to engage both binding sites and promote strand cleavage. The model rationalises published biochemical data and implies a novel role for the ERCC1 subunit of eukaryotic XPF complexes.
Organizational Affiliation:
Structural Biology Laboratory, London Research Institute, Cancer Research UK, London, UK.