Role of arginine-304 in the diphosphate-triggered active site closure mechanism of trichodiene synthase.
Vedula, L.S., Cane, D.E., Christianson, D.W.(2005) Biochemistry 44: 12719-12727
- PubMed: 16171386
- DOI: https://doi.org/10.1021/bi0510476
- Primary Citation of Related Structures:
2AEK, 2AEL, 2AET - PubMed Abstract:
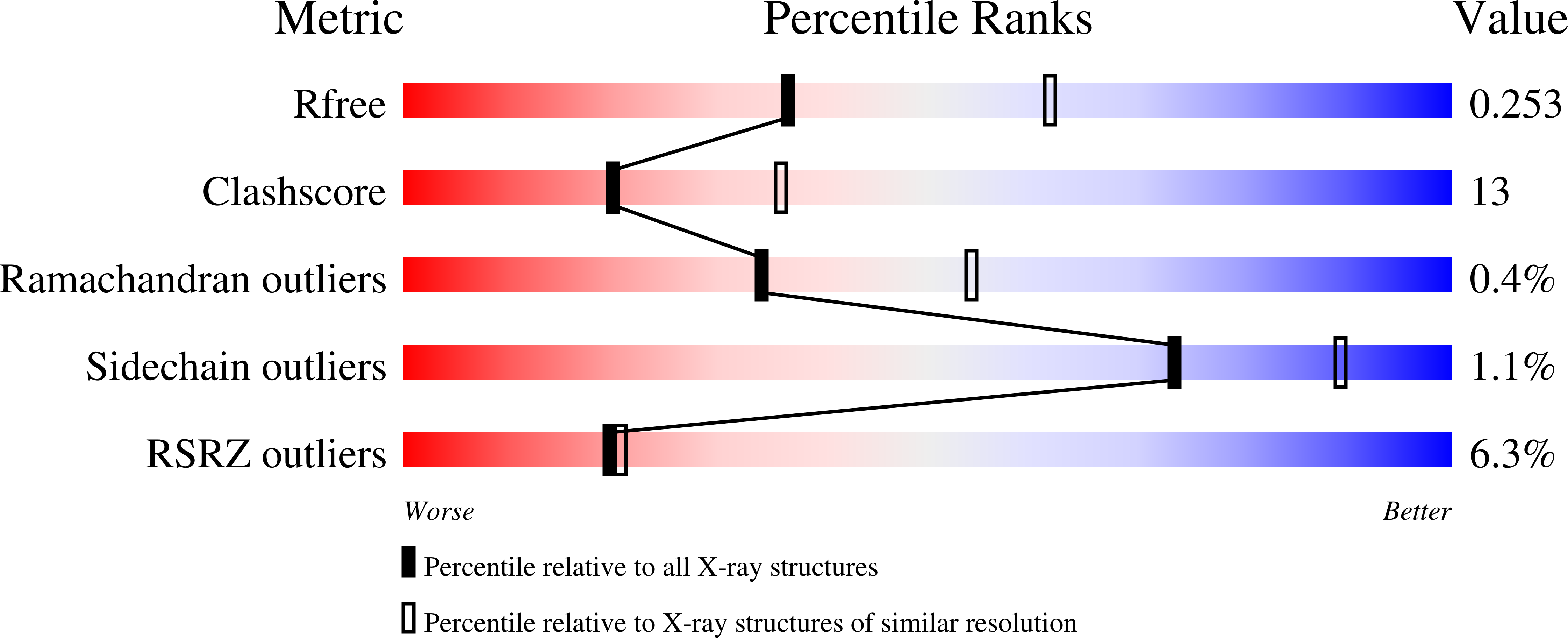
The X-ray crystal structures of R304K trichodiene synthase and its complexes with inorganic pyrophosphate (PP(i)) and aza analogues of the bisabolyl carbocation intermediate are reported. The R304K substitution does not cause large changes in the overall structure in comparison with the wild-type enzyme. The complexes with (R)- and (S)-azabisabolenes and PP(i) bind three Mg2+ ions, and each undergoes a diphosphate-triggered conformational change that caps the active site cavity. This conformational change is only slightly attenuated compared to that of the wild-type enzyme complexed with Mg2+(3)-PP(i), in which R304 donates hydrogen bonds to PP(i) and D101. In R304K trichodiene synthase, K304 does not engage in any hydrogen bond interactions in the unliganded state and it donates a hydrogen bond to only PP(i) in the complex with (R)-azabisabolene; K304 makes no hydrogen bond contacts in its complex with PP(i) and (S)-azabisabolene. Thus, although the R304-D101 hydrogen bond interaction stabilizes diphosphate-triggered active site closure, it is not required for Mg2+(3)-PP(i) binding. Nevertheless, since R304K trichodiene synthase generates aberrant cyclic terpenoids with a 5000-fold reduction in kcat/KM, it is clear that a properly formed R304-D101 hydrogen bond is required in the enzyme-substrate complex to stabilize the proper active site contour, which in turn facilitates cyclization of farnesyl diphosphate for the exclusive formation of trichodiene. Structural analysis of the R304K mutant and comparison with the monoterpene cyclase (+)-bornyl diphosphate synthase suggest that the significant loss in activity results from compromised activation of the PP(i) leaving group.
Organizational Affiliation:
Roy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6323, USA.