Structure of the regulatory apparatus of a calcium-dependent protein kinase (CDPK): a novel mode of calmodulin-target recognition.
Chandran, V., Stollar, E.J., Lindorff-Larsen, K., Harper, J.F., Chazin, W.J., Dobson, C.M., Luisi, B.F., Christodoulou, J.(2006) J Mol Biol 357: 400-410
- PubMed: 16430916
- DOI: https://doi.org/10.1016/j.jmb.2005.11.093
- Primary Citation of Related Structures:
2AAO - PubMed Abstract:
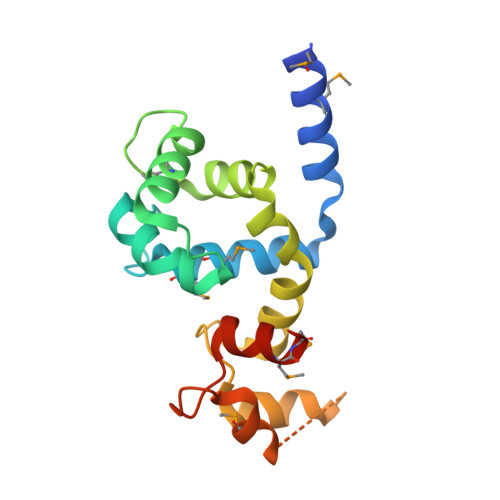
Calcium-dependent protein kinases (CDPKs) are a class of calcium-binding sensory proteins that are found in plants and certain protozoa, including the causative agent of malaria, Plasmodium falciparum. CDPKs have diverse regulatory functions, including involvement in the triggering of the lytic cycle of malarial infection. CDPKs contain an autoinhibitory junction (J) region whose calcium-dependent interaction with the tethered regulatory calmodulin-like domain (CaM-LD) activates the catalytic kinase domain. We report here the X-ray crystal structure of the J-CaM-LD region of CDPK from Arabidopsis thaliana (AtCPK1), determined to 2.0 A resolution using multiple-wavelength anomalous dispersion (MAD). The structure reveals a symmetric dimer of calcium-bound J-CaM-LD with domain-swap interactions, in which the J region of one protomer interacts extensively with the carboxy-terminal EF-hand domain (C-lobe) of the partner protomer. However, as the J-CaM-LD is monomeric in solution, the activated monomer was modelled to account for the intra-molecular recognition of the two domains. While the J-CaM-LD segment mimics certain aspects of target motif recognition by CaM other features are specific to CDPKs, in particular the combination of the strong interaction between the N and C-lobes of the CaM-LD and the exclusive use of only the C-lobe in the recognition of the covalently tethered target region. Combined with our previous observations showing that there is likely to be strong interactions between this tethered J region and the CaM-LD even at basal Ca(2+) concentrations, the new structural data indicate that the response to calcium of CDPKs is clearly unique among the CaM family.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK.