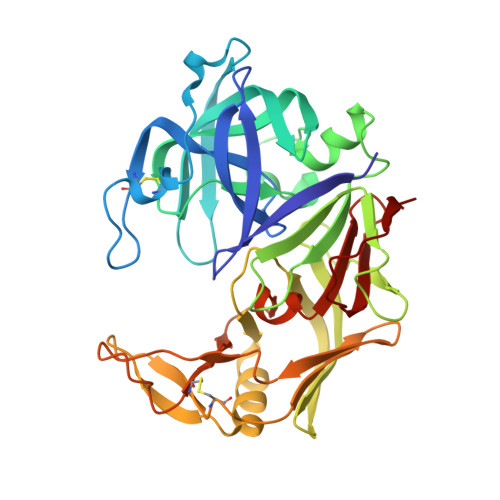
X-ray structures of Sap1 and Sap5: Structural comparison of the secreted aspartic proteinases from Candida albicans.
Borelli, C., Ruge, E., Lee, J.H., Schaller, M., Vogelsang, A., Monod, M., Korting, H.C., Huber, R., Maskos, K.(2008) Proteins 72: 1308-1319
- PubMed: 18384081
- DOI: https://doi.org/10.1002/prot.22021
- Primary Citation of Related Structures:
2QZW, 2QZX - PubMed Abstract:
Proteolytic activity is an important virulence factor for Candida albicans (C. albicans). It is attributed to the family of the secreted aspartic proteinases (Saps) from C. albicans with a minimum of 10 members. Saps show controlled expression and regulation for the individual stages of the infection process. Distinct isoenzymes can be responsible for adherence and tissue damage of local infections, while others cause systemic diseases. Earlier, only the structures of Sap2 and Sap3 were known. In our research, we have now succeeded in solving the X-ray crystal structures of the apoenzyme of Sap1 and Sap5 in complex with pepstatin A at 2.05 and 2.5 A resolution, respectively. With the structure of Sap1, we have completed the set of structures of isoenzyme subgroup Sap1-3. Of subgroup Sap4-6, the structure of the enzyme Sap5 is the first structure that has been described up to now. This facilitates comparison of structural details as well as inhibitor binding modes among the different subgroup members. Structural analysis reveals a highly conserved overall secondary structure of Sap1-3 and Sap5. However, Sap5 clearly differs from Sap1-3 by its electrostatic overall charge as well as through structural conformation of its entrance to the active site cleft. Design of inhibitors specific for Sap5 should concentrate on the S4 and S3 pockets, which significantly differ from Sap1-3 in size and electrostatic charge. Both Sap1 and Sap5 seem to play a major part in superficial Candida infections. Determination of the isoenzymes' structures can contribute to the development of new Sap-specific inhibitors for the treatment of superficial infections with a structure-based drug design program.
Organizational Affiliation:
Department of Dermatology and Allergy, Ludwig Maximilian University, 80337 Munich, Germany. claudia.borelli@med.uni-muenchen.de