Active site plasticity of endonuclease VIII: Conformational changes compensating for different substrates and mutations of critical residues
Golan, G., Zharkov, D.O., Grollman, A.P., Shoham, G.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Endonuclease VIII | C [auth A] | 262 | Escherichia coli | Mutation(s): 1 Gene Names: nei EC: 3.2.2 (PDB Primary Data), 4.2.99.18 (PDB Primary Data) | 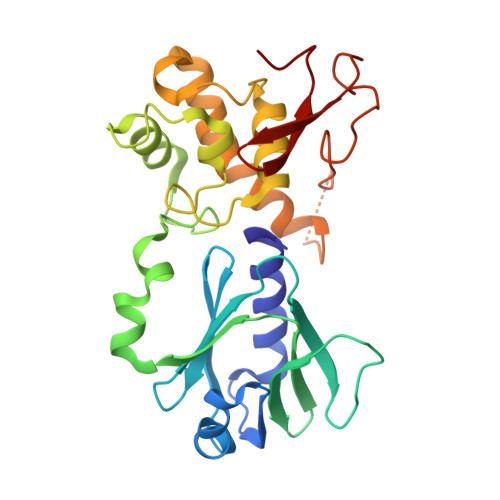 |
UniProt | |||||
Find proteins for P50465 (Escherichia coli (strain K12)) Explore P50465 Go to UniProtKB: P50465 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P50465 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-D(*GP*GP*CP*TP*TP*CP*AP*TP*CP*CP*TP*G)-3' | A [auth B] | 12 | N/A | 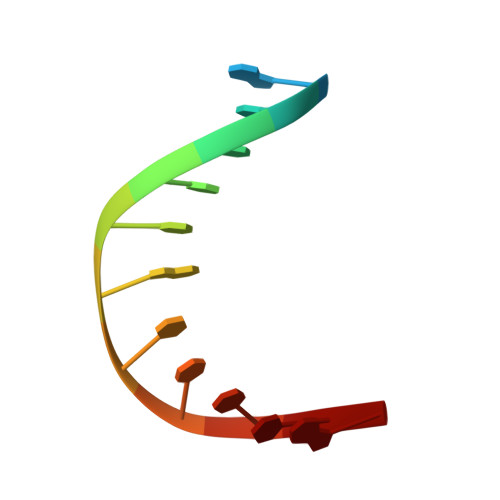 | |
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-D(P*CP*AP*GP*GP*AP*(PED)P*GP*AP*AP*GP*CP*C)-3' | B [auth C] | 12 | N/A | 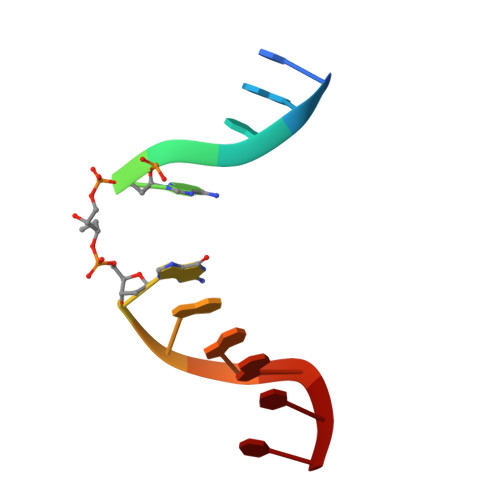 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | F [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | D [auth C], G [auth A], H [auth A], I [auth A], J [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | E [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 73.556 | α = 90 |
| b = 73.556 | β = 90 |
| c = 170.113 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| CNS | phasing |