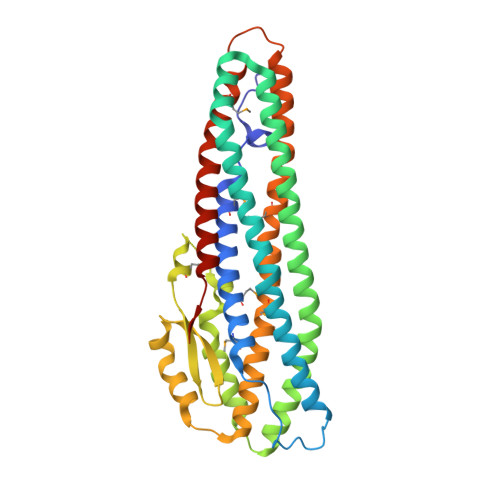
X-ray crystal structure of the B component of Hemolysin BL from Bacillus cereus
Madegowda, M., Eswaramoorthy, S., Burley, S.K., Swaminathan, S.(2008) Proteins 71: 534-540
- PubMed: 18175317
- DOI: https://doi.org/10.1002/prot.21888
- Primary Citation of Related Structures:
2NRJ - PubMed Abstract:
Bacillus cereus Hemolysin BL enterotoxin, a ternary complex of three proteins, is the causative agent of food poisoning and requires all three components for virulence. The X-ray structure of the binding domain of HBL suggests that it may form a pore similar to other soluble channel forming proteins. A putative pathway of pore formation is discussed.
Organizational Affiliation:
Biology Department, Brookhaven National Laboratory, Upton, New York 11973, USA.