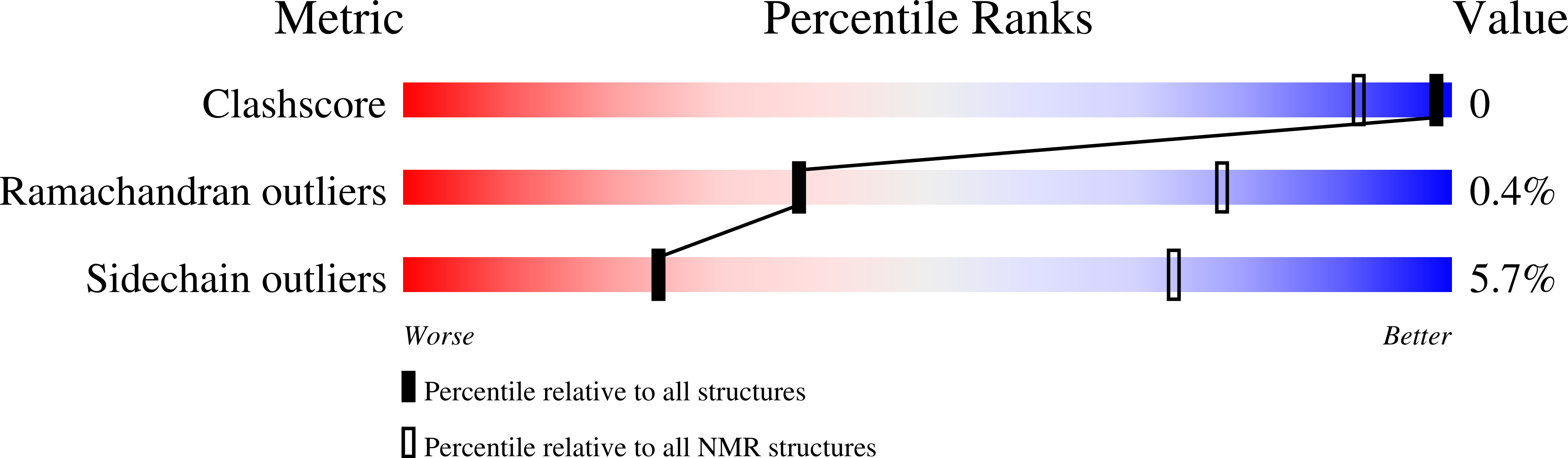
Solution structure of yeast Rpn9: insights into proteasome lid assembly.
Hu, Y., Wu, Y., Li, Q., Zhang, W., Jin, C.(2015) J Biol Chem 290: 6878-6889
- PubMed: 25631053
- DOI: https://doi.org/10.1074/jbc.M114.626762
- Primary Citation of Related Structures:
2MQW, 2MR3, 2MRI - PubMed Abstract:
The regulatory particle (RP) of the 26 S proteasome functions in preparing polyubiquitinated substrates for degradation. The lid complex of the RP contains an Rpn8-Rpn11 heterodimer surrounded by a horseshoe-shaped scaffold formed by six proteasome-COP9/CSN-initiation factor (PCI)-containing subunits. The PCI domains are essential for lid assembly, whereas the detailed molecular mechanisms remain elusive. Recent cryo-EM studies at near-atomic resolution provided invaluable information on the RP architecture in different functional states. Nevertheless, atomic resolution structural information on the RP is still limited, and deeper understanding of RP assembly mechanism requires further studies on the structures and interactions of individual subunits or subcomplexes. Herein we report the high-resolution NMR structures of the PCI-containing subunit Rpn9 from Saccharomyces cerevisiae. The 45-kDa protein contains an all-helical N-terminal domain and a C-terminal PCI domain linked via a semiflexible hinge. The N-terminal domain mediates interaction with the ubiquitin receptor Rpn10, whereas the PCI domain mediates interaction with the neighboring PCI subunit Rpn5. The Rpn9-Rpn5 interface highlights two structural motifs on the winged helix module forming a hydrophobic center surrounded by ionic pairs, which is a common pattern for all PCI-PCI interactions in the lid. The results suggest that divergence in surface composition among different PCI pairs may contribute to the modulation of lid assembly.
Organizational Affiliation:
From the Beijing Nuclear Magnetic Resonance Center, College of Chemistry and Molecular Engineering.