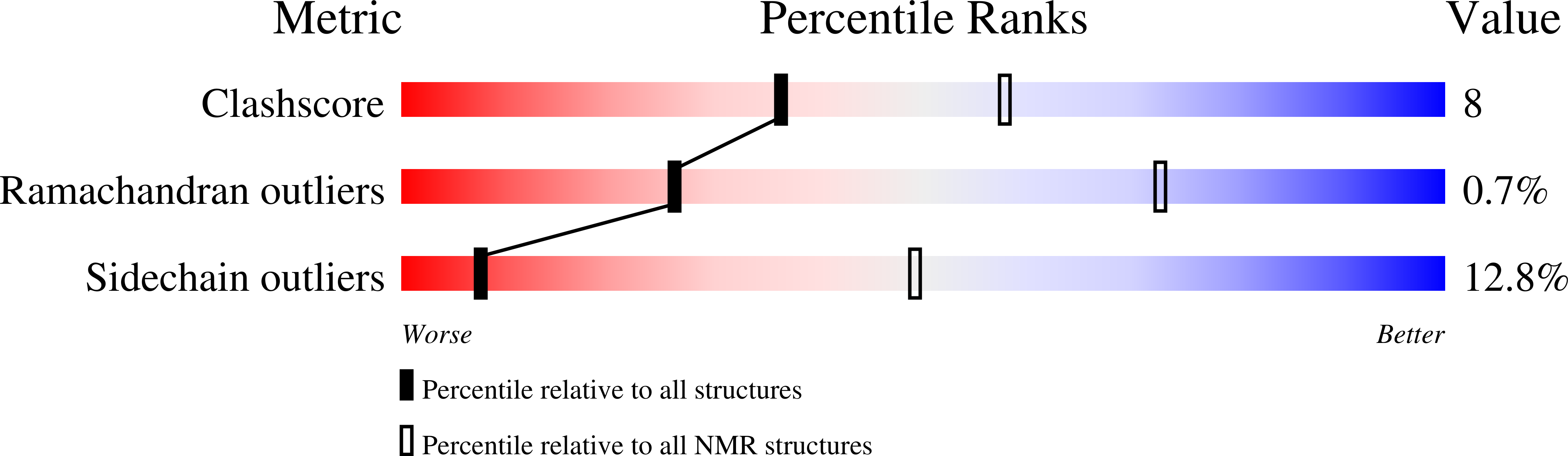
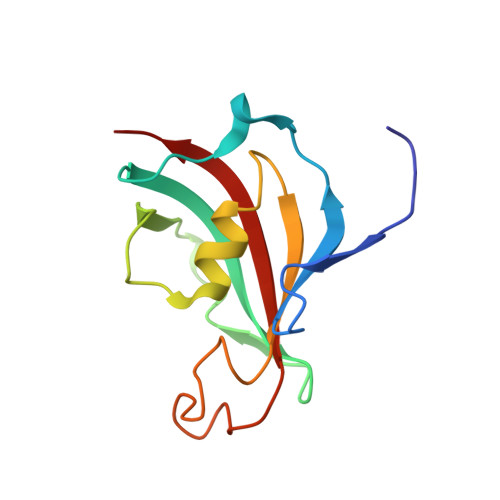
NMR assignments of the FK506-binding domain of FK506-binding protein 35 from Plasmodium vivax
Alag, R., Shin, J., Yoon, H.S.(2009) Biomol NMR Assign 3: 243-245
- PubMed: 19774494
- DOI: https://doi.org/10.1007/s12104-009-9185-1
- Primary Citation of Related Structures:
2KI3 - PubMed Abstract:
PvFKBP35 is a member of the FK506 binding protein family (FKBP) from Plasmodium vivax. The FK506-binding domain of PvFKBP35 shows a canonical peptidylprolyl cis-trans isomerase (PPIase) activity. To understand the role of PvFKBP35 in the parasite, we have performed NMR studies. Here, we report the assignment of the FK506-binding domain of PvFKBP35.
Organizational Affiliation:
School of Biological Science, Nanyang Technological University, Singapore, Singapore.