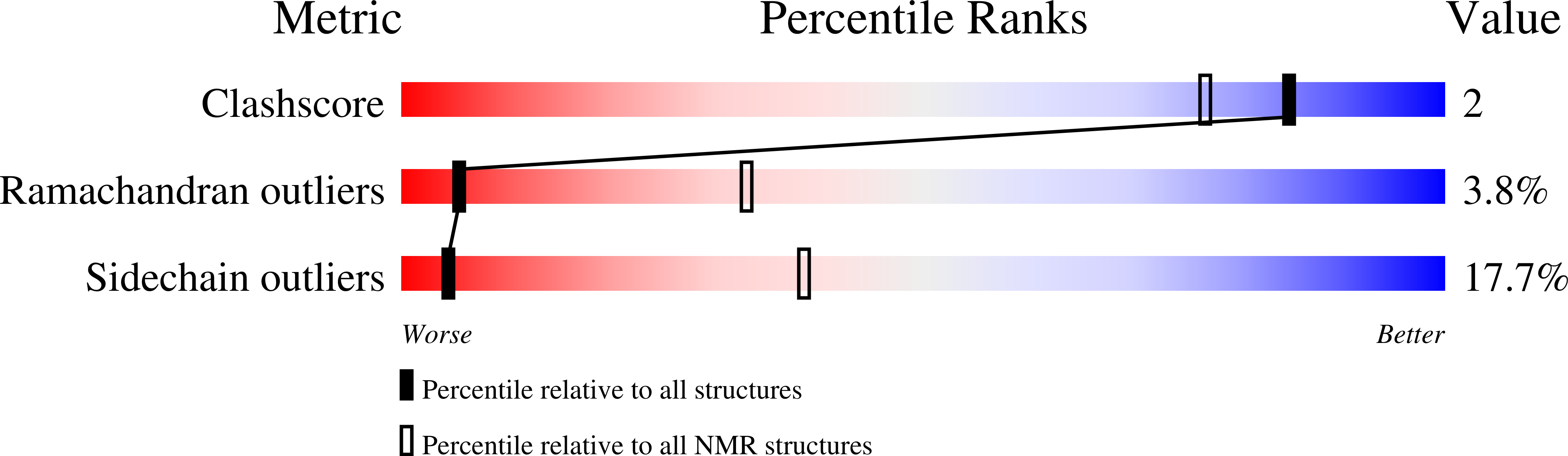The J-UNIO protocol for automated protein structure determination by NMR in solution.
Serrano, P., Pedrini, B., Mohanty, B., Geralt, M., Herrmann, T., Wuthrich, K.(2012) J Biomol NMR 53: 341-354
- PubMed: 22752932
- DOI: https://doi.org/10.1007/s10858-012-9645-2
- Primary Citation of Related Structures:
2KA7 - PubMed Abstract:
The J-UNIO (JCSG protocol using the software UNIO) procedure for automated protein structure determination by NMR in solution is introduced. In the present implementation, J-UNIO makes use of APSY-NMR spectroscopy, 3D heteronuclear-resolved [(1)H,(1)H]-NOESY experiments, and the software UNIO. Applications with proteins from the JCSG target list with sizes up to 150 residues showed that the procedure is highly robust and efficient. In all instances the correct polypeptide fold was obtained in the first round of automated data analysis and structure calculation. After interactive validation of the data obtained from the automated routine, the quality of the final structures was comparable to results from interactive structure determination. Special advantages are that the NMR data have been recorded with 6-10 days of instrument time per protein, that there is only a single step of chemical shift adjustments to relate the backbone signals in the APSY-NMR spectra with the corresponding backbone signals in the NOESY spectra, and that the NOE-based amino acid side chain chemical shift assignments are automatically focused on those residues that are heavily weighted in the structure calculation. The individual working steps of J-UNIO are illustrated with the structure determination of the protein YP_926445.1 from Shewanella amazonensis, and the results obtained with 17 JCSG targets are critically evaluated.
Organizational Affiliation:
Department of Molecular Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.