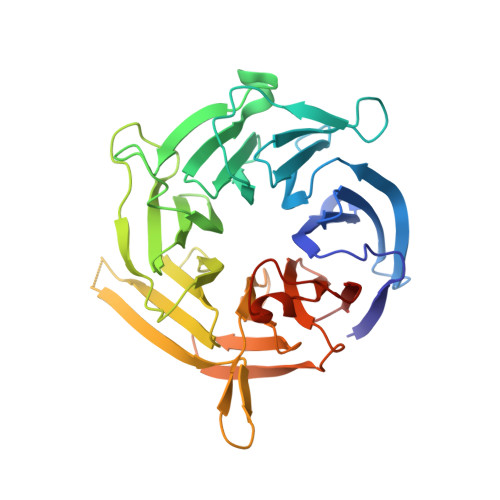
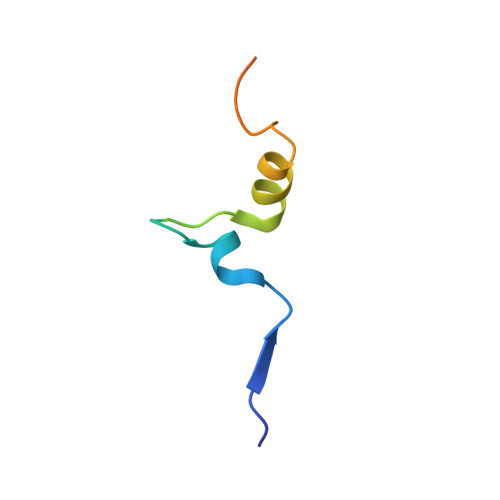
Structural analysis of Bub3 interactions in the mitotic spindle checkpoint.
Larsen, N.A., Al-Bassam, J., Wei, R.R., Harrison, S.C.(2007) Proc Natl Acad Sci U S A 104: 1201-1206
- PubMed: 17227844
- DOI: https://doi.org/10.1073/pnas.0610358104
- Primary Citation of Related Structures:
2I3S, 2I3T - PubMed Abstract:
The Mad3/BubR1, Mad2, Bub1, and Bub3 proteins are gatekeepers for the transition from metaphase to anaphase. Mad3 from Saccharomyces cerevisiae has homology to Bub1 but lacks a corresponding C-terminal kinase domain. Mad3 forms a stable heterodimer with Bub3. Negative-stain electron microscopy shows that Mad3 is an extended molecule (approximately 200 A long), whereas Bub3 is globular. The Gle2-binding-sequence (GLEBS) motifs found in Mad3 and Bub1 are necessary and sufficient for interaction with Bub3. The calorimetrically determined dissociation constants for GLEBS-motif peptides and Bub3 are approximately 5 microM. Crystal structures of these peptides with Bub3 show that the interactions for Mad3 and Bub1 are similar and mutually exclusive. In both structures, the GLEBS peptide snakes along the top surface of the beta-propeller, forming an extensive interface. Mutations in either protein that disrupt the interface cause checkpoint deficiency and chromosome instability. We propose that the structure imposed on the GLEBS segment by its association with Bub3 enables recruitment to unattached kinetochores.
Organizational Affiliation:
Jack Eileen Connors Structural Biology Laboratory, and Howard Hughes Medical Institute, Harvard Medical School, 250 Longwood Avenue, Boston, MA 02115, USA.