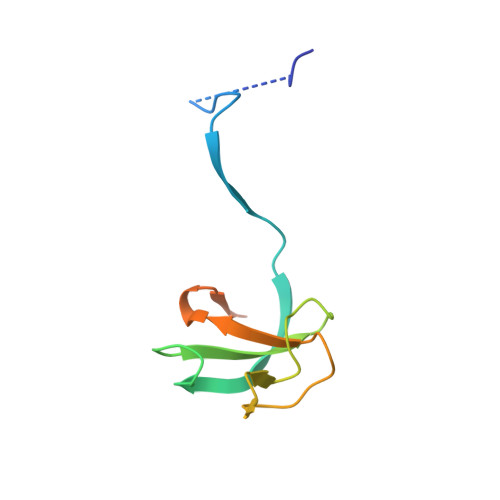
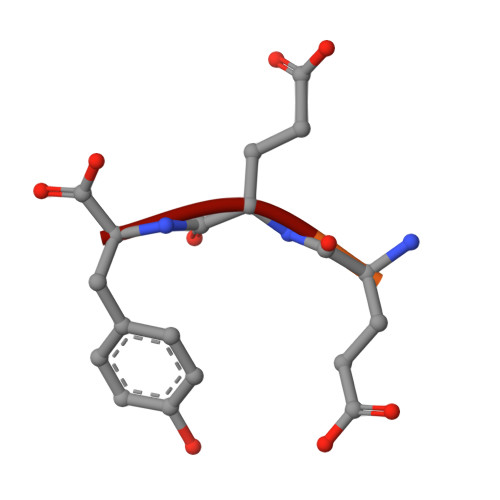
Key interaction modes of dynamic +TIP networks.
Honnappa, S., Okhrimenko, O., Jaussi, R., Jawhari, H., Jelesarov, I., Winkler, F.K., Steinmetz, M.O.(2006) Mol Cell 23: 663-671
- PubMed: 16949363
- DOI: https://doi.org/10.1016/j.molcel.2006.07.013
- Primary Citation of Related Structures:
2HKN, 2HKQ, 2HL3, 2HL5 - PubMed Abstract:
Dynamic microtubule plus-end tracking protein (+TIP) networks are implicated in all functions of microtubules, but the molecular determinants of their interactions are largely unknown. Here, we have explored key binding modes of +TIPs by analyzing the interactions between selected CAP-Gly, EB-like, and carboxy-terminal EEY/F-COO(-) sequence motifs. X-ray crystallography and biophysical binding studies demonstrate that the beta2-beta3 loop of CAP-Gly domains determines EB-like motif binding specificity. They further show how CAP-Gly domains serve as recognition domains for EEY/F-COO(-) motifs, which represent characteristic and functionally important sequence elements in EB, CLIP-170, and alpha-tubulin. Our findings provide a molecular basis for understanding the modular interaction modes between alpha-tubulin, CLIPs, EB proteins, and the dynactin-dynein motor complex and suggest that multiple low-affinity binding sites in different combinations control dynamic +TIP networks at microtubule ends. They further offer insights into the structural consequences of genetic CAP-Gly domain defects found in severe human disorders.
Organizational Affiliation:
Biomolecular Research, Structural Biology, Paul Scherrer Institut, CH-5232 Villigen PSI, Switzerland.