Structures of Trypanosoma cruzi dihydroorotate dehydrogenase complexed with substrates and products: atomic resolution insights into mechanisms of dihydroorotate oxidation and fumarate reduction
Inaoka, D.K., Sakamoto, K., Shimizu, H., Shiba, T., Kurisu, G., Nara, T., Aoki, T., Kita, K., Harada, S.(2008) Biochemistry 47: 10881-10891
- PubMed: 18808149
- DOI: https://doi.org/10.1021/bi800413r
- Primary Citation of Related Structures:
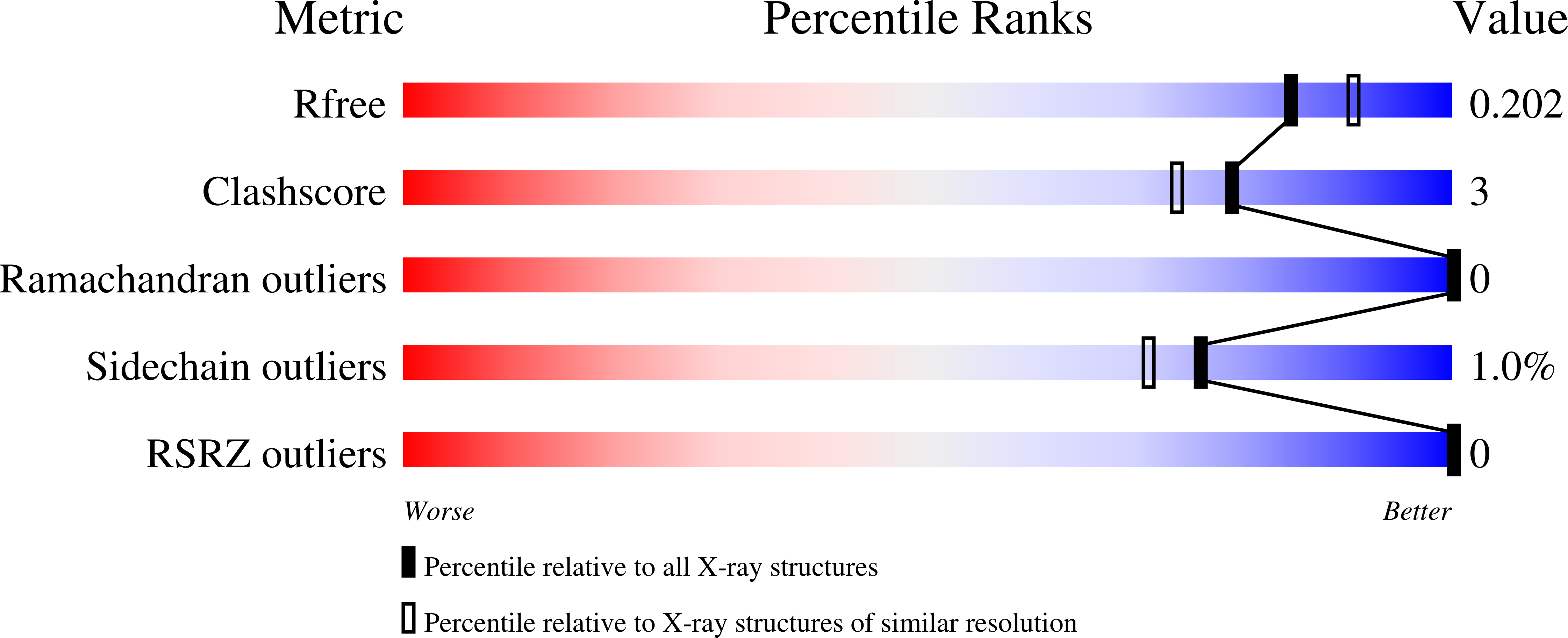
2E6D - PubMed Abstract:
Dihydroorotate dehydrogenase (DHOD) from Trypanosoma cruzi (TcDHOD) is a member of family 1A DHOD that catalyzes the oxidation of dihydroorotate to orotate (first half-reaction) and then the reduction of fumarate to succinate (second half-reaction) in the de novo pyrimidine biosynthesis pathway. The oxidation of dihydroorotate is coupled with the reduction of FMN, and the reduced FMN converts fumarate to succinate in the second half-reaction. TcDHOD are known to be essential for survival and growth of T. cruzi and a validated drug target. The first-half reaction mechanism of the family 1A DHOD from Lactococcus lactis has been extensively investigated on the basis of kinetic isotope effects, mutagenesis and X-ray structures determined for ligand-free form and in complex with orotate, the product of the first half-reaction. In this report, we present crystal structures of TcDHOD in the ligand-free form and in complexes with an inhibitor, physiological substrates and products of the first and second half-reactions. These ligands bind to the same active site of TcDHOD, which is consistent with the one-site ping-pong Bi-Bi mechanism demonstrated by kinetic studies for family 1A DHODs. The binding of ligands to TcDHOD does not cause any significant structural changes to TcDHOD, and both reduced and oxidized FMN cofactors are in planar conformation, which indicates that the reduction of the FMN cofactor with dihydroorotate produces anionic reduced FMN. Therefore, they should be good models for the enzymatic reaction pathway of TcDHOD, although orotate and fumarate bind to TcDHOD with the oxidized FMN and dihydroorotate with the reduced FMN in the structures determined here. Cys130, which was identified as the active site base for family 1A DHOD (Fagan, R. L., Jensen, K. F., Bjornberg, O., and Palfey, B. A. (2007) Biochemistry 46, 4028-4036.), is well located for abstracting a proton from dihydroorotate C5 and transferring it to outside water molecules. The bound fumarate is in a twisted conformation, which induces partial charge separation represented as C 2 (delta-) and C 3 (delta+). Because of this partial charge separation, the thermodynamically favorable reduction of fumarate with reduced FMN seems to proceed in the way that C 2 (delta-) accepts a proton from Cys130 and C 3 (delta+) a hydride (or a hydride equivalent) from reduced FMN N 5 in TcDHOD.
Organizational Affiliation:
Department of Biomedical Chemistry, Graduate School of Medicine, The University of Tokyo, Tokyo 113-0033, Japan. danielken@m.u-tokyo.ac.jp