In vivo, kinetic, and structural analysis of the development of ritonavir resistance
Clemente, J.C., Stow, L.R., Janka, L.K., Jeung, J.A., Desai, K.A., Govindasamy, L., Agbandje-McKenna, M., McKenna, R., Goodenow, M.M., Dunn, B.M.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HIV-1 protease | A [auth B], B [auth A] | 99 | Human immunodeficiency virus 1 | Mutation(s): 10 | 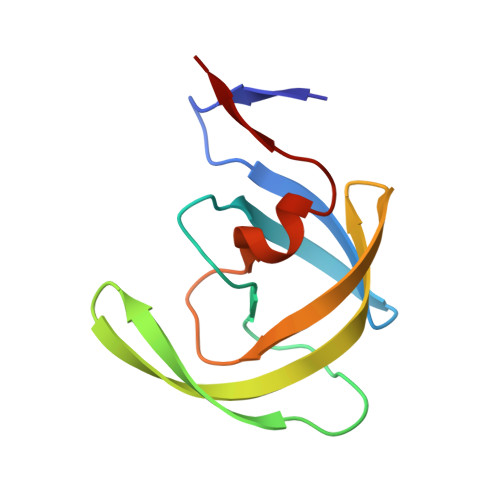 |
UniProt | |||||
Find proteins for P03367 (Human immunodeficiency virus type 1 group M subtype B (isolate BRU/LAI)) Explore P03367 Go to UniProtKB: P03367 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P03367 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MK1 Query on MK1 | C [auth B] | N-[2(R)-HYDROXY-1(S)-INDANYL]-5-[(2(S)-TERTIARY
BUTYLAMINOCARBONYL)-4(3-PYRIDYLMETHYL)PIPERAZINO]-4(S)-HYDROXY-2(R)-PHENYLMETHYLPENTANAMIDE C36 H47 N5 O4 CBVCZFGXHXORBI-PXQQMZJSSA-N |  | ||
| Binding Affinity Annotations | |||
|---|---|---|---|
| ID | Source | Binding Affinity | |
| MK1 | BindingDB: 2B7Z | Ki: min: 0.05, max: 398 (nM) from 35 assay(s) | |
| Kd: 1.07 (nM) from 1 assay(s) | |||
| IC50: min: 0.36, max: 370 (nM) from 18 assay(s) | |||
| -TΔS: min: -7.40e+1, max: -2.80e+1 (kJ/mol) from 27 assay(s) | |||
| ΔH: min: -3.34e+1, max: 35.11 (kJ/mol) from 27 assay(s) | |||
| ΔG: min: -6.31e+1, max: -3.85e+1 (kJ/mol) from 28 assay(s) | |||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 61.831 | α = 90 |
| b = 61.831 | β = 90 |
| c = 84.033 | γ = 120 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| CNS | refinement |
| PDB_EXTRACT | data extraction |
| CNS | phasing |