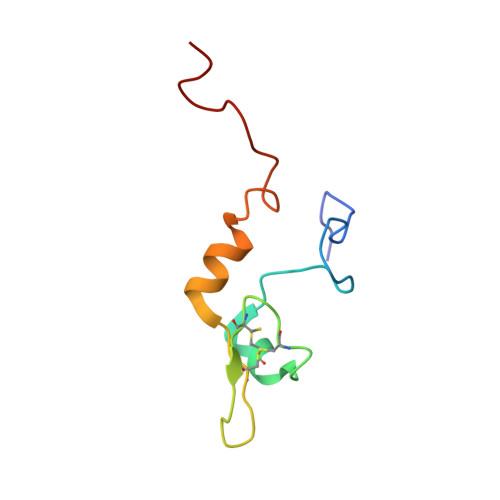
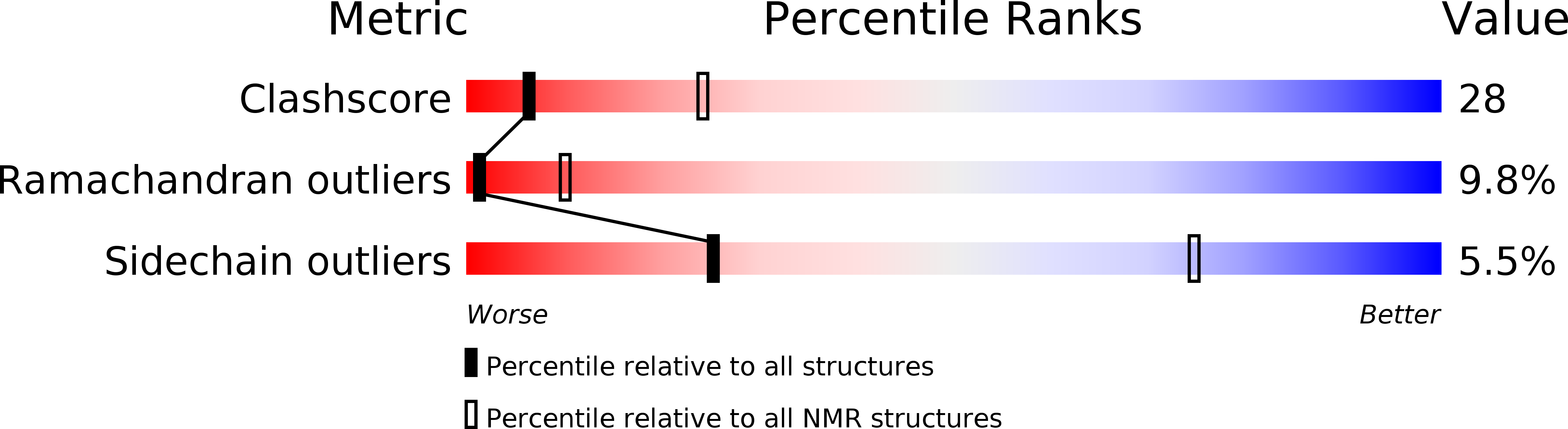Structure of the C-terminal domain from Trypanosoma brucei variant surface glycoprotein MITat1.2
Chattopadhyay, A., Jones, N.G., Nietlispach, D., Nielsen, P.R., Voorheis, H.P., Mott, H.R., Carrington, M.(2004) J Biol Chem 280: 7228-7235
- PubMed: 15557330
- DOI: https://doi.org/10.1074/jbc.M410787200
- Primary Citation of Related Structures:
1XU6 - PubMed Abstract:
The variant surface glycoprotein (VSG) of African trypanosomes has a structural role in protecting other cell surface proteins from effector molecules of the mammalian immune system and also undergoes antigenic variation necessary for a persistent infection in a host. Here we have reported the solution structure of a VSG type 2 C-terminal domain from MITat1.2, completing the first structure of both domains of a VSG. The isolated C-terminal domain is a monomer in solution and forms a novel fold, which commences with a short alpha-helix followed by a single turn of 3(10)-helix and connected by a short loop to a small anti-parallel beta-sheet and then a longer alpha-helix at the C terminus. This compact domain is flanked by two unstructured regions. The structured part of the domain contains 42 residues, and the core comprises 2 disulfide bonds and 2 hydrophobic residues. These cysteines and hydrophobic residues are conserved in other VSGs, and we have modeled the structures of two further VSG C-terminal domains using the structure of MITat1.2. The models suggest that the overall structure of the core is conserved in the different VSGs but that the C-terminal alpha-helix is of variable length and depends on the presence of charged residues. The results provided evidence for a conserved tertiary structure for all the type 2 VSG C-terminal domains, indicated that VSG dimers form through interactions between N-terminal domains, and showed that the selection pressure for sequence variation within a conserved tertiary structure acts on the whole of the VSG molecule.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, United Kingdom.