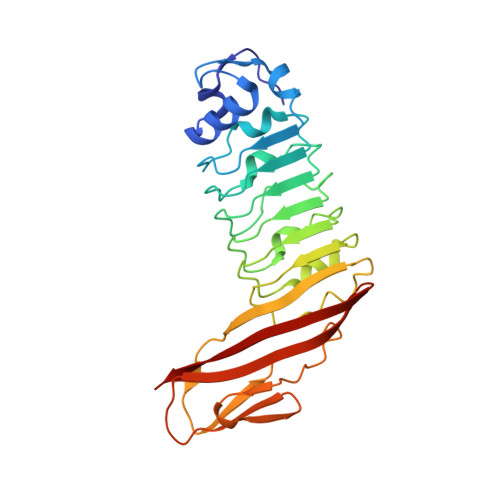
Structure of internalin C from Listeria monocytogenes.
Ooi, A., Hussain, S., Seyedarabi, A., Pickersgill, R.W.(2006) Acta Crystallogr D Biol Crystallogr 62: 1287-1293
- PubMed: 17057330
- DOI: https://doi.org/10.1107/S0907444906026746
- Primary Citation of Related Structures:
1XEU - PubMed Abstract:
The crystal structure of internalin C (InlC) from Listeria monocytogenes has been determined at 2.0 A resolution. Several observations implicate InlC in infection: inlC has the same transcriptional activator as other virulence genes, it is only present in pathogenic Listeria strains and an inlC deletion mutant is significantly less virulent. While the extended concave receptor-binding surfaces of the leucine-rich repeat (LRR) domains of internalins A and B have aromatic clusters involved in receptor binding, the corresponding surface of InlC is smaller, flatter and more hydrophilic, suggesting that InlC may be involved in weak or transient associations with receptors; this may help explain why no receptor has yet been discovered for InlC. In contrast, the Ig-like domain, to which the LRR domain is fused, has surface aromatics that may be of functional importance, possibly being involved in binding to the surface of the bacteria or in receptor binding.
Organizational Affiliation:
School of Biological and Chemical Sciences, Queen Mary, University of London, Mile End Road, London E1 4NS, England.