Harvesting the high-hanging fruit: the structure of the YdeN gene product from Bacillus subtilis at 1.8 angstroms resolution.
Janda, I., Devedjiev, Y., Cooper, D., Chruszcz, M., Derewenda, U., Gabrys, A., Minor, W., Joachimiak, A., Derewenda, Z.S.(2004) Acta Crystallogr D Biol Crystallogr 60: 1101-1107
- PubMed: 15159570
- DOI: https://doi.org/10.1107/S0907444904007188
- Primary Citation of Related Structures:
1UXO - PubMed Abstract:
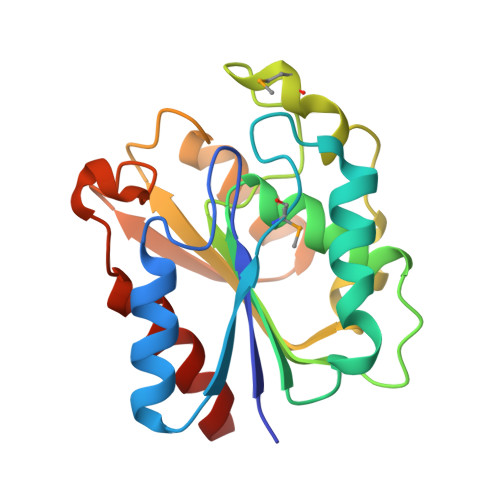
High-throughput (HT) protein crystallography is severely impeded by the relatively low success rate of protein crystallization. Proteins whose structures are not solved in the HT pipeline owing to attrition in any phase of the project are referred to as the high-hanging fruit, in contrast to those proteins that yielded good-quality crystals and crystal structures, which are referred to as low-hanging fruit. It has previously been shown that proteins that do not crystallize in the wild-type form can have their surfaces engineered by site-directed mutagenesis in order to create patches of low conformational entropy that are conducive to forming intermolecular interactions. The application of this method to selected proteins from the Bacillus subtilis genome which failed to crystallize in the HT mode is now reported. In this paper, the crystal structure of the product of the YdeN gene is reported. Of three prepared double mutants, i.e. E124A/K127A, E167A/E169A and K88A/Q89A, the latter gave high-quality crystals and the crystal structure was solved by SAD at 1.8 angstroms resolution. The protein is a canonical alpha/beta hydrolase, with an active site that is accessible to solvent.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22908-0736, USA.