Solution Structure of Spheniscin, a {Beta}-Defensin from the Penguin Stomach
Landon, C., Thouzeau, C., Labbe, H., Bulet, P., Vovelle, F.(2004) J Biol Chem 279: 30433
- PubMed: 15123713
- DOI: https://doi.org/10.1074/jbc.M401338200
- Primary Citation of Related Structures:
1UT3 - PubMed Abstract:
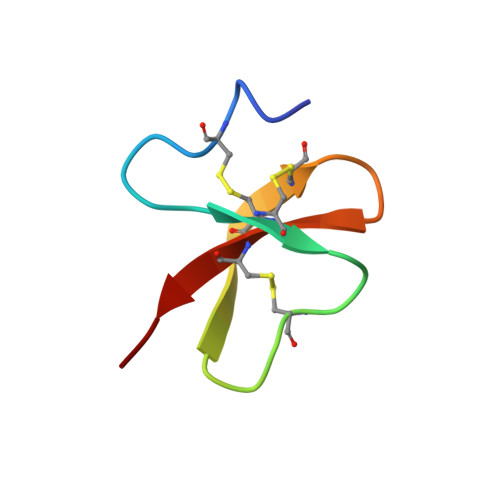
Recently two beta-defensins, named spheniscins, have been isolated from the stomach content of the king penguin (Aptenodytes patagonicus), which is capable of preserving food for several weeks during egg incubation (Thouzeau, C., Le Maho, Y., Froget, G., Sabatier, L., Le Bohec, C., Hoffmann, J. A., and Bulet, P. (2003) J. Biol. Chem. 278, 51053-51058). It has been proposed that, in combination with other antimicrobial peptides, spheniscins may be involved in this long term preservation of food in the bird's stomach. To draw some structure/function features, the three-dimensional structure in aqueous solution of the most abundant spheniscin (Sphe-2) was determined by two-dimensional NMR and molecular modeling techniques. The overall fold of Sphe-2 includes a three-stranded antiparallel beta-sheet stabilized by three disulfide bridges with a pairing typical of beta-defensins. In addition, the N-terminal segment shows helical features on most structures. Sphe-2 is highly cationic, and its surface displays a hydrophobic patch. Comparative modeling revealed that this patch is preserved in avian defensins. The activity of Sphe-2 against a pathogenic Gram-positive strain was retained in vitro in the conditions of osmolarity found in penguin stomach content and also in different salt concentrations and compositions up to those reported for seawater. Comparison with structurally related mammalian beta-defensins showed that the hydrophobic patch is not preserved in mammalian beta-defensins and that the high cationicity of Sphe-2 is presumably the critical factor for its retained activity in high salt concentrations. Such peculiarities, in addition to a broad activity spectrum, suggest that penguin defensins may represent interesting probes for the design of highly efficient antibiotics to fight off pathogens that develop in relatively salt-rich body fluids.
Organizational Affiliation:
Centre de Biophysique Moléculaire, CNRS UPR 4301, Université d'Orléans, rue Charles Sadron, 45071 Orléans 2, France.