The Structure of the C-C Bond Hydrolase MhpC Provides Insights into its Catalytic Mechanism
Dunn, G., Montgomery, M.G., Mohammed, F., Coker, A., Cooper, J.B., Robertson, T., Garcia, J.-L., Bugg, T.D.H., Wood, S.P.(2005) J Mol Biol 346: 253-265
- PubMed: 15663942
- DOI: https://doi.org/10.1016/j.jmb.2004.11.033
- Primary Citation of Related Structures:
1U2E - PubMed Abstract:
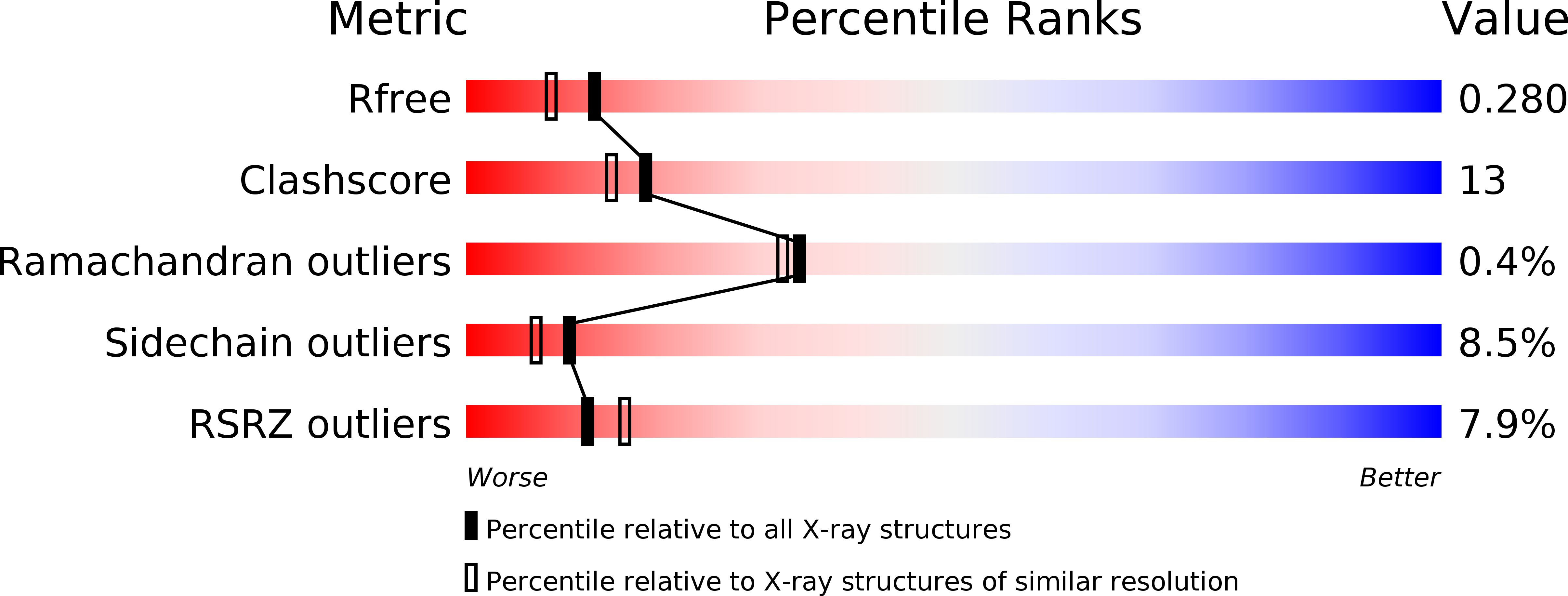
2-Hydroxy-6-ketonona-2,4-diene-1,9-dioic acid 5,6-hydrolase (MhpC) is a 62 kDa homodimeric enzyme of the phenylpropionate degradation pathway of Escherichia coli. The 2.1 A resolution X-ray structure of the native enzyme determined from orthorhombic crystals confirms that it is a member of the alpha/beta hydrolase fold family, comprising eight beta-strands interconnected by loops and helices. The 2.8 A resolution structure of the enzyme co-crystallised with the non-hydrolysable substrate analogue 2,6-diketo-nona-1,9-dioic acid (DKNDA) confirms the location of the active site in a buried channel including Ser110, His263 and Asp235, postulated contributors to a serine protease-like catalytic triad in homologous enzymes. It appears that the ligand binds in two separate orientations. In the first, the C6 keto group of the inhibitor forms a hemi-ketal adduct with the Ser110 side-chain, the C9 carboxylate group interacts, via the intermediacy of a water molecule, with Arg188 at one end of the active site, while the C1 carboxylate group of the inhibitor comes close to His114 at the other end. In the second orientation, the C1 carboxylate group binds at the Arg188 end of the active site and the C9 carboxylate group at the His114 end. These arrangements implicated His114 or His263 as plausible contributors to catalysis of the initial enol/keto tautomerisation of the substrate but lack of conservation of His114 amongst related enzymes and mutagenesis results suggest that His263 is the residue involved. Variability in the quality of the electron density for the inhibitor amongst the eight molecules of the crystal asymmetric unit appears to correlate with alternative positions for the side-chain of His114. This might arise from half-site occupation of the dimeric enzyme and reflect the apparent dissociation of approximately 50% of the keto intermediate from the enzyme during the catalytic cycle.
Organizational Affiliation:
Department of Biomolecular Science, University of Southampton, Bassett Crescent East, Southampton, SO16 7PX, UK.