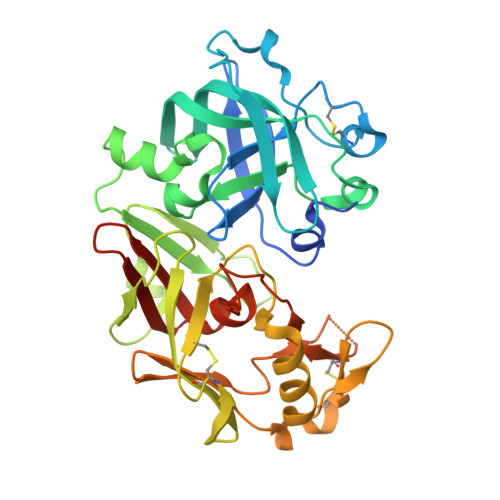
Crystal structure of an activation intermediate of cathepsin e
Ostermann, N., Gerhartz, B., Worpenberg, S., Trappe, J., Eder, J.(2004) J Mol Biol 342: 889-899
- PubMed: 15342244
- DOI: https://doi.org/10.1016/j.jmb.2004.07.073
- Primary Citation of Related Structures:
1TZS - PubMed Abstract:
Cathepsin E is an intracellular, non-lysosomal aspartic protease expressed in a variety of cells and tissues. The protease has proposed physiological roles in antigen presentation by the MHC class II system, in the biogenesis of the vasoconstrictor peptide endothelin, and in neurodegeneration associated with brain ischemia and aging. Cathepsin E is the only A1 aspartic protease that exists as a homodimer with a disulfide bridge linking the two monomers. Like many other aspartic proteases, it is synthesized as a zymogen which is catalytically inactive towards its natural substrates at neutral pH and which auto-activates in an acidic environment. Here we report the crystal structure of an activation intermediate of human cathepsin E at 2.35A resolution. The overall structure follows the general fold of aspartic proteases of the A1 family, and the intermediate shares many features with the intermediate 2 on the proposed activation pathway of aspartic proteases like pepsin C and cathepsin D. The pro-sequence is cleaved from the protease and remains stably associated with the mature enzyme by forming the outermost sixth strand of the interdomain beta-sheet. However, different from these other aspartic proteases the pro-sequence of cathepsin E remains intact after cleavage from the mature enzyme. In addition, the active site of cathepsin E in the crystal is occupied by N-terminal amino acid residues of the mature protease in the non-primed binding site and by an artificial N-terminal extension of the pro-sequence from a neighboring molecule in the primed site. The crystal structure of the cathepsin E/pro-sequence complex, therefore, provides further insight into the activation mechanism of aspartic proteases.
Organizational Affiliation:
Protease Platform, Novartis Institutes for BioMedical Research, CH-4002 Basel, Switzerland.