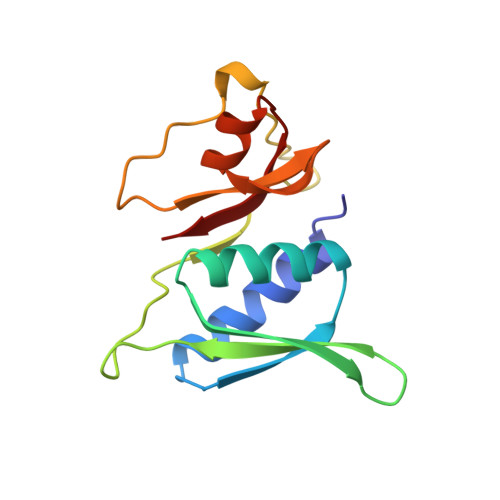
Structural evidence for specific S8-RNA and S8-protein interactions within the 30S ribosomal subunit: ribosomal protein S8 from Bacillus stearothermophilus at 1.9 A resolution.
Davies, C., Ramakrishnan, V., White, S.W.(1996) Structure 4: 1093-1104
- PubMed: 8805594
- DOI: https://doi.org/10.1016/s0969-2126(96)00115-3
- Primary Citation of Related Structures:
1SEI - PubMed Abstract:
Prokaryotic ribosomal protein S8 is an important RNA-binding protein that occupies a central position within the small ribosomal subunit. It interacts extensively with 16S rRNA and is crucial for the correct folding of the central domain of the rRNA. S8 also controls the synthesis of several ribosomal proteins by binding to mRNA. It binds specifically to very similar sites in the two RNA molecules.
Organizational Affiliation:
Department of Microbiology, Duke University Medical Center, Durham, NC 27710, USA.