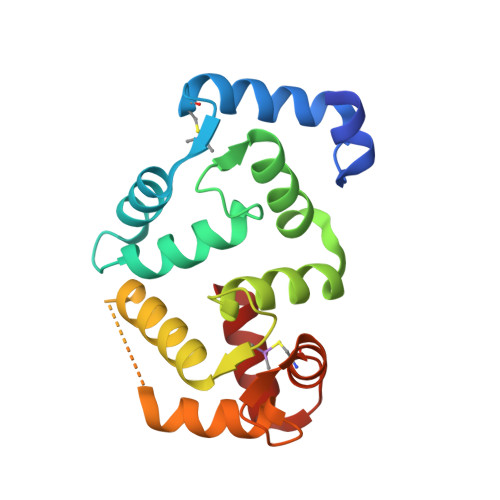
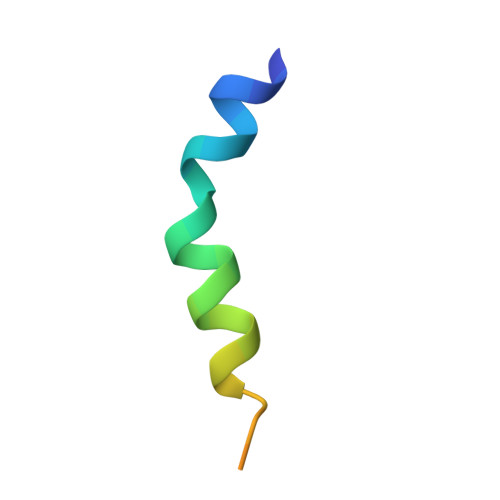
Structural insights into the functional interaction of KChIP1 with Shal-type K(+) channels.
Zhou, W., Qian, Y., Kunjilwar, K., Pfaffinger, P.J., Choe, S.(2004) Neuron 41: 573-586
- PubMed: 14980206
- DOI: https://doi.org/10.1016/s0896-6273(04)00045-5
- Primary Citation of Related Structures:
1S6C - PubMed Abstract:
Four Kv channel-interacting proteins (KChIP1 through KChIP4) interact directly with the N-terminal domain of three Shal-type voltage-gated potassium channels (Kv4.1, Kv4.2, and Kv4.3) to modulate cell surface expression and function of Kv4 channels. Here we report a 2.0 Angstrom crystal structure of the core domain of KChIP1 (KChIP1*) in complex with the N-terminal fragment of Kv4.2 (Kv4.2N30). The complex reveals a clam-shaped dimeric assembly. Four EF-hands from each KChIP1 form each shell of the clam. The N-terminal end of Kv4.2 forming an alpha helix (alpha1) and the C-terminal alpha helix (H10) of KChIP1 are enclosed nearly coaxially by these shells. As a result, the H10 of KChIP1 and alpha1 of Kv4.2 mediate interactions between these two molecules, structurally reminiscent of the interactions between calmodulin and its target peptides. Site-specific mutagenesis combined with functional characterization shows that those interactions mediated by alpha1 and H10 are essential to the modulation of Kv4.2 by KChIPs.
Organizational Affiliation:
Division of Neuroscience, Baylor College of Medicine, Houston, TX 77030 USA.