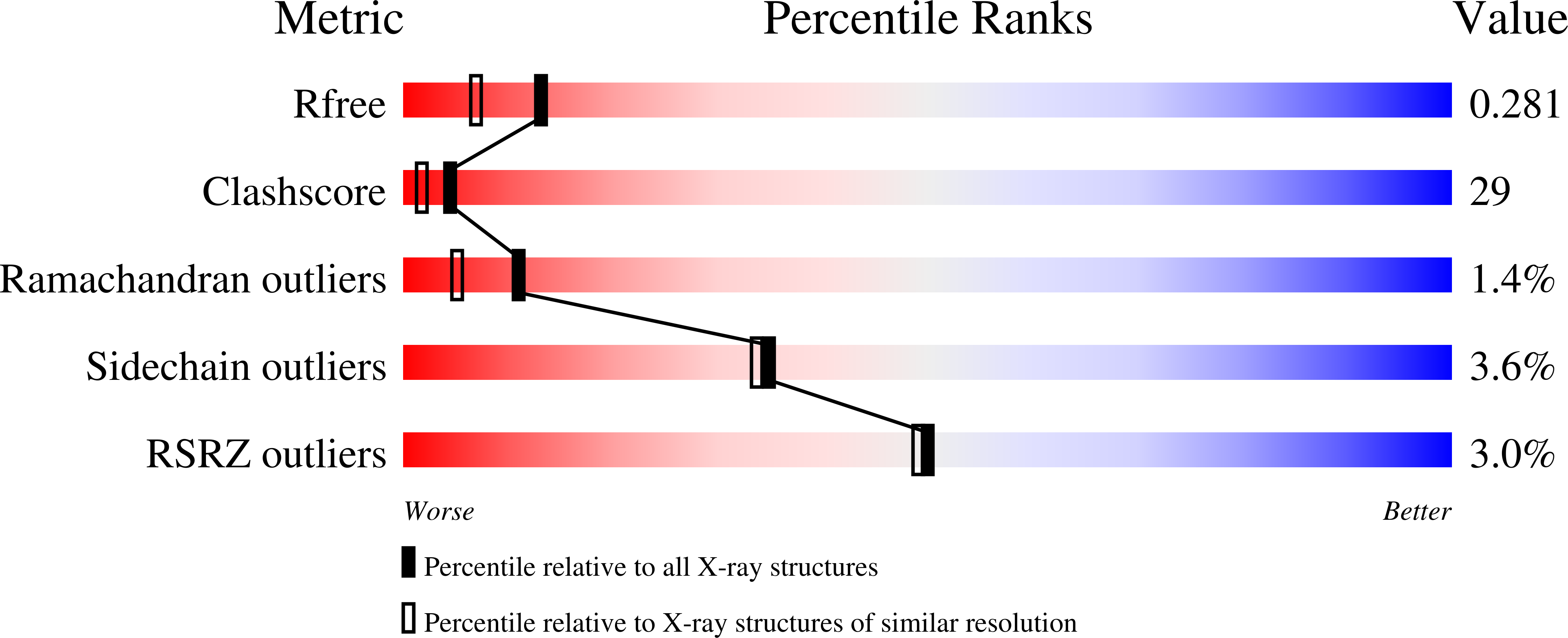
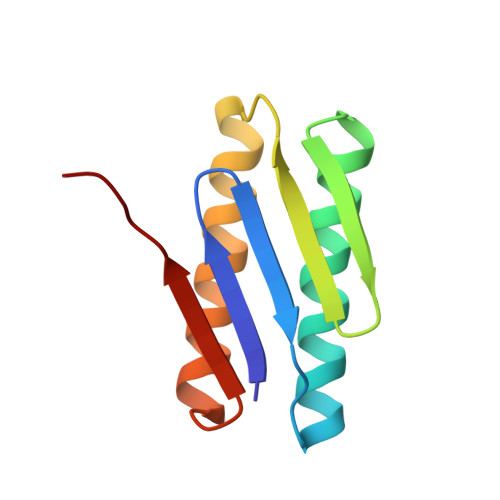
Crystal structure of TM1457 from Thermotoga maritima.
Shin, D.H., Lou, Y., Jancarik, J., Yokota, H., Kim, R., Kim, S.H.(2005) J Struct Biol 152: 113-117
- PubMed: 16242963
- DOI: https://doi.org/10.1016/j.jsb.2005.08.008
- Primary Citation of Related Structures:
1S12 - PubMed Abstract:
The crystal structure of a hypothetical protein, TM1457, from Thermotoga maritima has been determined at 2.0A resolution. TM1457 belongs to the DUF464 family (57 members) for which there is no known function. The structure shows that it is composed of two helices in contact with one side of a five-stranded beta-sheet. Two identical monomers form a pseudo-dimer in the asymmetric unit. There is a large cleft between the first alpha-helix and the second beta-strand. This cleft may be functionally important, since the two highly conserved motifs, GHA and VCAXV(S/T), are located around the cleft. A structural comparison of TM1457 with known protein structures shows the best hit with another hypothetical protein, Ybl001C from Saccharomyces cerevisiae, though they share low structural similarity. Therefore, TM1457 still retains a unique topology and reveals a novel fold.
Organizational Affiliation:
College of Pharmacy, Ewha Womans University, Seoul 120-750, Korea.