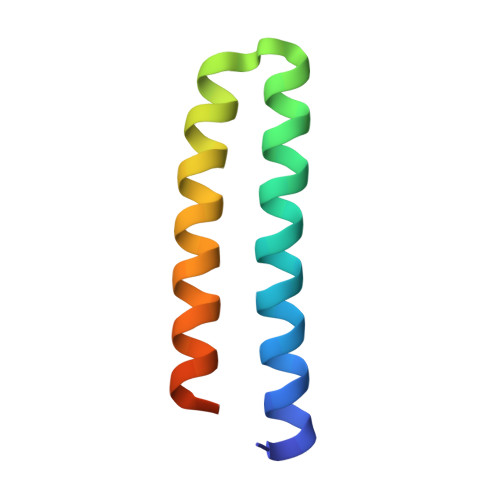
Structure of the ColE1 rop protein at 1.7 A resolution
Banner, D.W., Kokkinidis, M., Tsernoglou, D.(1987) J Mol Biol 196: 657-675
- PubMed: 3681971
- DOI: https://doi.org/10.1016/0022-2836(87)90039-8
- Primary Citation of Related Structures:
1ROP - PubMed Abstract:
Structural details of the Rop protein from plasmid ColE1 are presented, with a description of the X-ray crystal structure determination and refinement at a nominal resolution of 1.7 A. The 63 amino acid protein is a dimer. Each monomer consists almost entirely of two alpha helices, the whole molecule forming a highly regular four-alpha-helix bundle. This may be approximated by a four-stranded rope with a radius of 7.0 A, a left-handed helical twist and a pitch of 172.5 A. The packing constraints for this novel type of coiled-coil structure are given. The protein acts in the control of plasmid replication via regulation of an RNA-RNA interaction in a manner not yet understood in atomic detail.
Organizational Affiliation:
EMBL, Heidelberg, FRG.