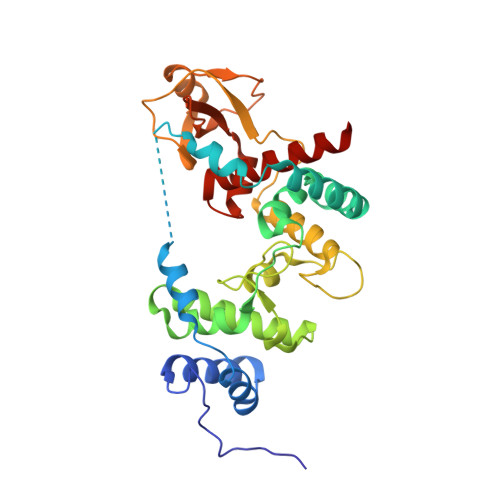
Crystal structure of Escherichia coli lytic transglycosylase Slt35 reveals a lysozyme-like catalytic domain with an EF-hand.
van Asselt, E.J., Dijkstra, A.J., Kalk, K.H., Takacs, B., Keck, W., Dijkstra, B.W.(1999) Structure 7: 1167-1180
- PubMed: 10545329
- DOI: https://doi.org/10.1016/s0969-2126(00)80051-9
- Primary Citation of Related Structures:
1QUS - PubMed Abstract:
Lytic transglycosylases are bacterial muramidases that catalyse the cleavage of the beta- 1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) in peptidoglycan with concomitant formation of a 1,6-anhydrobond in the MurNAc residue. These muramidases play an important role in the metabolism of the bacterial cell wall and might therefore be potential targets for the rational design of antibacterial drugs. One of the lytic transglycosylases is Slt35, a naturally occurring soluble fragment of the outer membrane bound lytic transglycosylase B (MltB) from Escherichia coli.
Organizational Affiliation:
BIOSON Research Institute, Laboratory of Biophysical Chemistry Groningen University, Nijenborgh 4, 9747 AG, Groningen, The Netherlands.