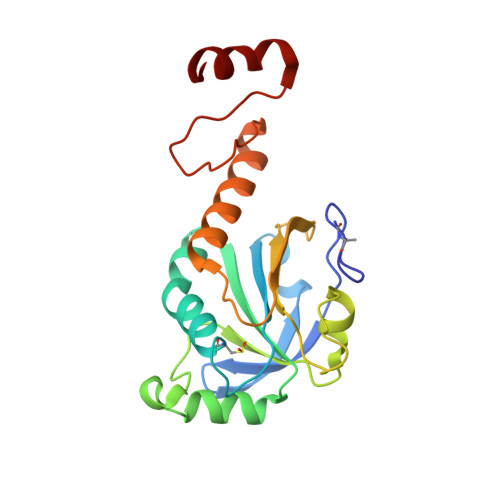
Crystal Structure of Decameric 2-Cys Peroxiredoxin from Human Erythrocytes at 1.7 A Resolution.
Schroder, E., Littlechild, J.A., Lebedev, A.A., Errington, N., Vagin, A.A., Isupov, M.N.(2000) Structure 8: 605
- PubMed: 10873855
- DOI: https://doi.org/10.1016/s0969-2126(00)00147-7
- Primary Citation of Related Structures:
1QMV - PubMed Abstract:
The peroxiredoxins (Prxs) are an emerging family of multifunctional enzymes that exhibit peroxidase activity in vitro, and in vivo participate in a range of cellular processes known to be sensitive to reactive oxygen species. Thioredoxin peroxidase B (TPx-B), a 2-Cys type II Prx from erythrocytes, promotes potassium efflux and down-regulates apoptosis and the recruitment of monocytes by endothelial tissue.
Organizational Affiliation:
Schools of Chemistry and Biological Sciences, University of Exeter, Exeter, EX4 4QD, UK. eschrode@ex.ac.uk