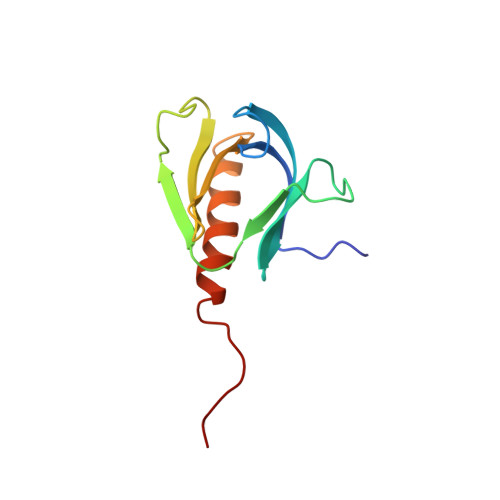
Solution structure of a pleckstrin-homology domain.
Yoon, H.S., Hajduk, P.J., Petros, A.M., Olejniczak, E.T., Meadows, R.P., Fesik, S.W.(1994) Nature 369: 672-675
- PubMed: 8208296
- DOI: https://doi.org/10.1038/369672a0
- Primary Citation of Related Structures:
1PLS - PubMed Abstract:
Pleckstrin, the major protein kinase C substrate of platelets, contains domains of about 100 amino acids at the amino and carboxy termini that have been found in a number of proteins, including serine/threonine kinases, GTPase-activating proteins, phospholipases and cytoskeletal proteins. These conserved sequences, termed pleckstrin-homology (PH) domains, are thought to be involved in signal transduction. But the details of the function and binding partners of the PH domains have not been characterized. Here we report the solution structure of the N-terminal pleckstrin-homology domain of pleckstrin determined using heteronuclear three-dimensional nuclear magnetic resonance spectroscopy. The structure consists of an up-and-down beta-barrel of seven antiparallel beta-strands and a C-terminal amphiphilic alpha-helix that caps one end of the barrel. The overall topology of the domain is similar to that of the retinol-binding protein family of structures.
Organizational Affiliation:
Pharmaceutical Discovery Division, Abbott Laboratories, Abbott Park, Illinois 60064.