RECOGNITION OF ACCESSORY PROTEIN MOTIFS BY THE GAMMA-ADAPTIN EAR DOMAIN OF GGA3
MILLER, G.J., MATTERA, R., BONIFACINO, J.S., HURLEY, J.H.(2003) Nat Struct Biol 10: 599-606
- PubMed: 12858162
- DOI: https://doi.org/10.1038/nsb953
- Primary Citation of Related Structures:
1P4U - PubMed Abstract:
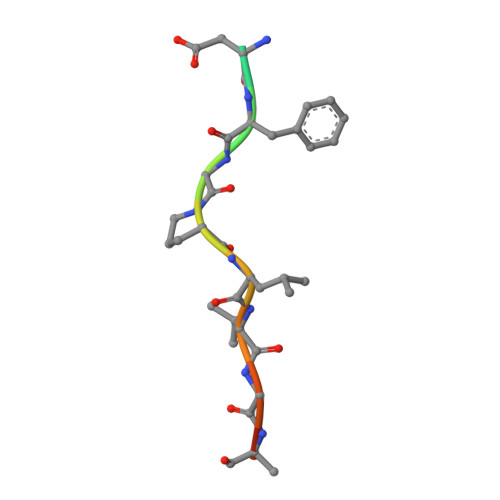
Adaptor proteins load transmembrane protein cargo into transport vesicles and serve as nexuses for the formation of large multiprotein complexes on the nascent vesicles. The gamma-adaptin ear (GAE) domains of the AP-1 adaptor protein complex and the GGA adaptor proteins recruit accessory proteins to these multiprotein complexes by binding to a hydrophobic motif. We determined the structure of the GAE domain of human GGA3 in complex with a peptide based on the DFGPLV sequence of the accessory protein Rabaptin-5 and refined it at a resolution of 2.2 A. The leucine and valine residues of the peptide are partly buried in two contiguous shallow, hydrophobic depressions. The anchoring phenylalanine is buried in a deep pocket formed by the aliphatic portions of two conserved arginine residues, along with an alanine and a proline, illustrating the unusual function of a cluster of basic residues in binding a hydrophobic motif.
Organizational Affiliation:
Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland 20892, USA.