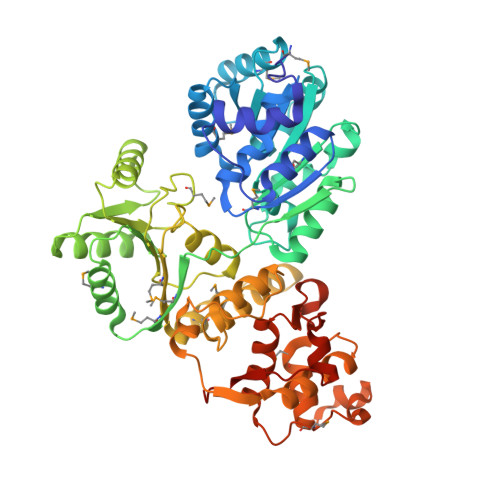
High-resolution structure of the E. coli RecQ helicase catalytic core
Bernstein, D.A., Zittel, M.C., Keck, J.L.(2003) EMBO J 22: 4910-4921
- PubMed: 14517231
- DOI: https://doi.org/10.1093/emboj/cdg500
- Primary Citation of Related Structures:
1OYW, 1OYY - PubMed Abstract:
RecQ family helicases catalyze critical genome maintenance reactions in bacterial and eukaryotic cells, playing key roles in several DNA metabolic processes. Mutations in recQ genes are linked to genome instability and human disease. To define the physical basis of RecQ enzyme function, we have determined a 1.8 A resolution crystal structure of the catalytic core of Escherichia coli RecQ in its unbound form and a 2.5 A resolution structure of the core bound to the ATP analog ATPgammaS. The RecQ core comprises four conserved subdomains; two of these combine to form its helicase region, while the others form unexpected Zn(2+)-binding and winged-helix motifs. The structures reveal the molecular basis of missense mutations that cause Bloom's syndrome, a human RecQ-associated disease. Finally, based on findings from the structures, we propose a mechanism for RecQ activity that could explain its functional coordination with topoisomerase III.
Organizational Affiliation:
Department of Biomolecular Chemistry, 550 Medical Science Center, 1300 University Avenue, University of Wisconsin Medical School, Madison, WI 53706-1532, USA.