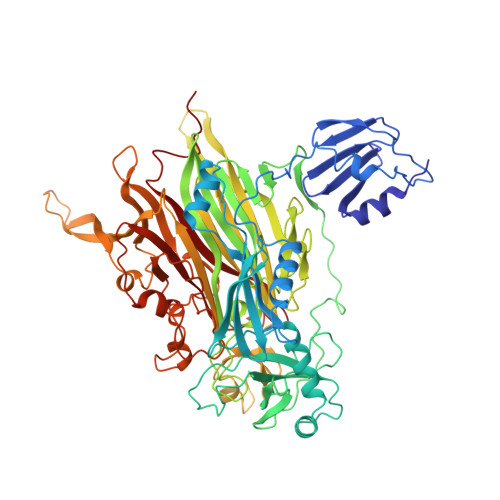
Crystal structure of a quinoenzyme: copper amine oxidase of Escherichia coli at 2 A resolution.
Parsons, M.R., Convery, M.A., Wilmot, C.M., Yadav, K.D., Blakeley, V., Corner, A.S., Phillips, S.E., McPherson, M.J., Knowles, P.F.(1995) Structure 3: 1171-1184
- PubMed: 8591028
- DOI: https://doi.org/10.1016/s0969-2126(01)00253-2
- Primary Citation of Related Structures:
1OAC - PubMed Abstract:
Copper amine oxidases are a ubiquitous and novel group of quinoenzymes that catalyze the oxidative deamination of primary amines to the corresponding aldehydes, with concomitant reduction of molecular oxygen to hydrogen peroxide. The enzymes are dimers of identical 70-90 kDa subunits, each of which contains a single copper ion and a covalently bound cofactor formed by the post-translational modification of a tyrosine side chain to 2,4,5-trihydroxyphenylalanine quinone (TPQ).
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Leeds, UK.