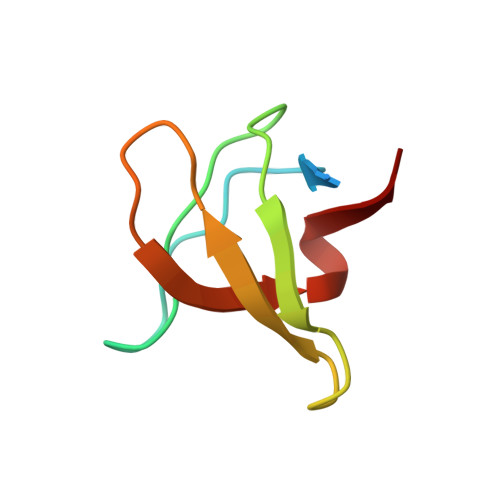
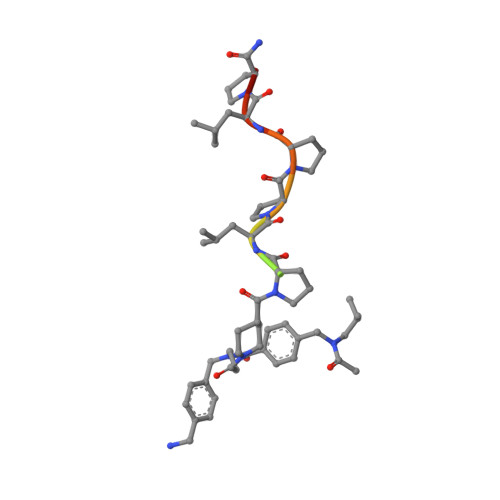
Molecular basis for the binding of SH3 ligands with non-peptide elements identified by combinatorial synthesis.
Feng, S., Kapoor, T.M., Shirai, F., Combs, A.P., Schreiber, S.L.(1996) Chem Biol 3: 661-670
- PubMed: 8807900
- DOI: https://doi.org/10.1016/s1074-5521(96)90134-9
- Primary Citation of Related Structures:
1NLO, 1NLP - PubMed Abstract:
Protein-structure-based combinatorial chemistry has recently been used to discover several ligands containing non-peptide binding elements to the Src SH3 domain. The encoded library used has the form Cap-M1-M2-M3-PLPPLP, in which the Cap and Mi's are composed of a diverse set of organic monomers. The PLPPLP portion provided a structural bias directing the non-peptide fragment Cap-M1-M2-M3 to the SH3 specificity pocket. Fifteen ligands were selected from > 1.1 million distinct compounds. The structural basis for selection was unknown.
Organizational Affiliation:
Howard Hughes Medical Institute, Department of Chemistry and Chemical Biology, Harvard University, MA 02138, USA.