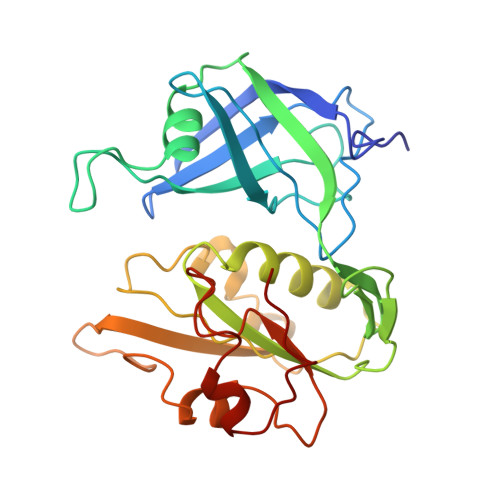
Crystal structure of NADH-cytochrome b5 reductase from pig liver at 2.4 A resolution.
Nishida, H., Inaka, K., Yamanaka, M., Kaida, S., Kobayashi, K., Miki, K.(1995) Biochemistry 34: 2763-2767
- PubMed: 7893687
- DOI: https://doi.org/10.1021/bi00009a004
- Primary Citation of Related Structures:
1NDH - PubMed Abstract:
The three-dimensional structure of NADH-cytochrome b5 reductase from pig liver microsomes has been determined at 2.4 A resolution by X-ray crystallography. The molecular structure reveals two domains, the FAD binding domain and the NADH domain. A large cleft lies between these two domains and contains the binding site for the FAD prosthetic group. The backbone structure of the FAD binding domain has a great similarity to that of ferredoxin-NADP+ reductase [Karplus, P. A., Daniels, M. J., & Herriott, J. R. (1991) Science 251, 60-65], in spite of the relatively low sequence homology (about 15%) between the two enzymes. On the other hand, the structure of the NADH domain has several structural differences from that of the NADP+ domain of ferredoxin-NADP+ reductase. The size of the cleft between the two domains is larger in NADH-cytochrome b5 reductase than in ferredoxin-NADP+ reductase, which may be responsible for the observed difference in the nucleotide accessibility in the two enzymes.
Organizational Affiliation:
Research Laboratory of Resources Utilization, Tokyo Institute of Technology, Yokohama, Japan.