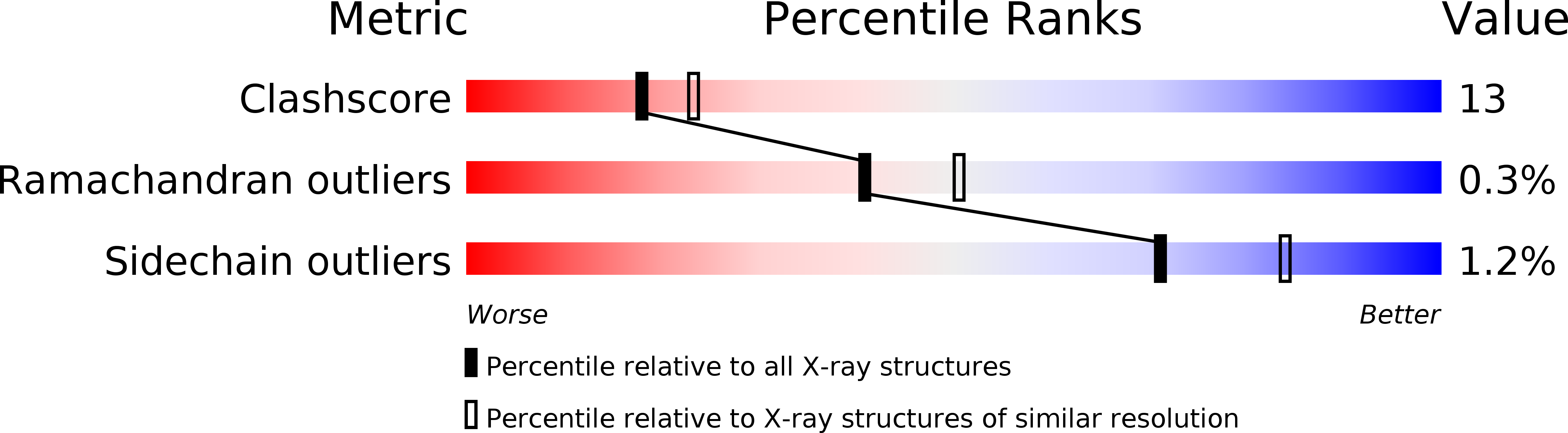Porcine Carbonyl Reductase: Structural Basis for a Functional Monomer in Short-Chain Dehydrogenases/Reductases
Ghosh, D., Sawicki, M., Pletnev, V., Erman, M., Duax, W.L., Ohno, S., Nakajin, S.(2001) J Biol Chem 276: 18457-18463
- PubMed: 11279087
- DOI: https://doi.org/10.1074/jbc.M100538200
- Primary Citation of Related Structures:
1N5D - PubMed Abstract:
Porcine testicular carbonyl reductase (PTCR) belongs to the short chain dehydrogenases/reductases (SDR) superfamily and catalyzes the NADPH-dependent reduction of ketones on steroids and prostaglandins. The enzyme shares nearly 85% sequence identity with the NADPH-dependent human 15-hydroxyprostaglandin dehydrogenase/carbonyl reductase. The tertiary structure of the enzyme at 2.3 A reveals a fold characteristic of the SDR superfamily that uses a Tyr-Lys-Ser triad as catalytic residues, but exhibits neither the functional homotetramer nor the homodimer that distinguish all SDRs. It is the first known monomeric structure in the SDR superfamily. In PTCR, which is also active as a monomer, a 41-residue insertion immediately before the catalytic Tyr describes an all-helix subdomain that packs against interfacial helices, eliminating the four-helix bundle interface conserved in the superfamily. An additional anti-parallel strand in the PTCR structure also blocks the other strand-mediated interface. These novel structural features provide the basis for the scaffolding of one catalytic site within a single molecule of the enzyme.
Organizational Affiliation:
Department of Structural Biology, Hauptman-Woodward Medical Research Institute, Buffalo, New York 14203, USA.