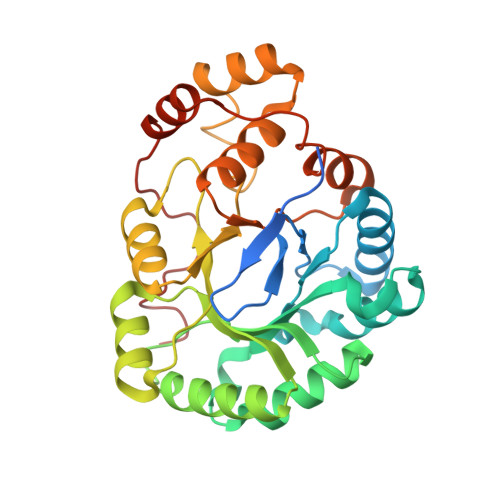
Crystal structure of Escherichia coli DkgA, a broad-specificity aldo-keto reductase.
Jeudy, S., Monchois, V., Maza, C., Claverie, J.M., Abergel, C.(2006) Proteins 62: 302-307
- PubMed: 16284956
- DOI: https://doi.org/10.1002/prot.20710
- Primary Citation of Related Structures:
1MZR
Organizational Affiliation:
Information Génomique et Structurale, CNRS, Marseille, France.