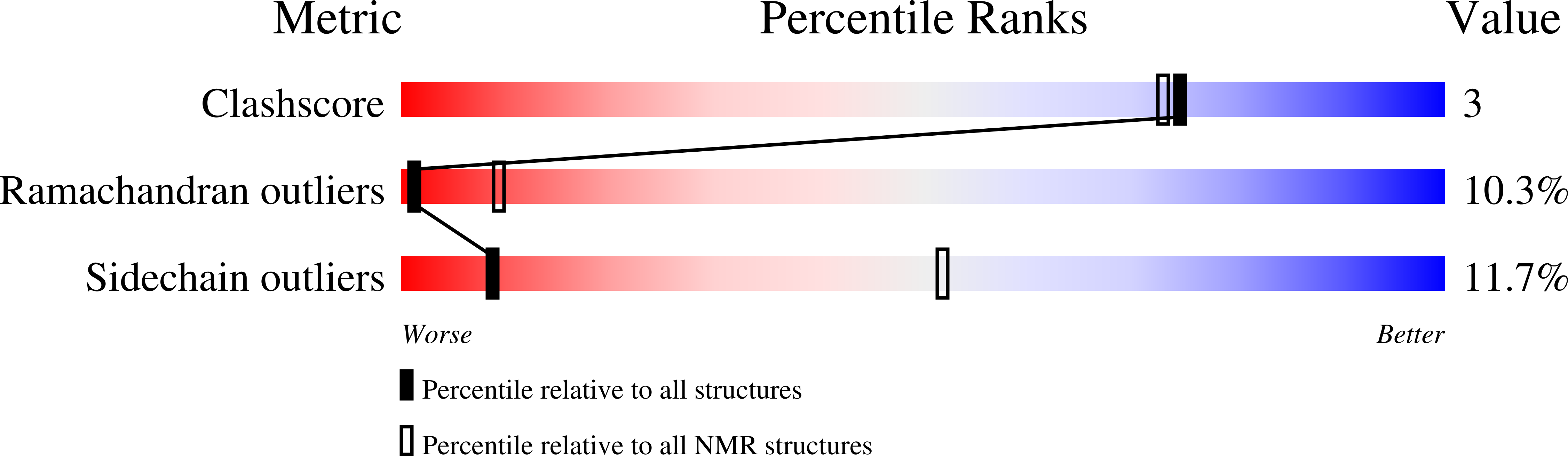Relaxation-based structure refinement and backbone molecular dynamics of the Dynein motor domain-associated light chain
Wu, H., Blackledge, M., Maciejewski, M.W., Mullen, G.P., King, S.M.(2003) Biochemistry 42: 57-71
- PubMed: 12515539
- DOI: https://doi.org/10.1021/bi026762j
- Primary Citation of Related Structures:
1M9L - PubMed Abstract:
The light chain 1 (LC1) polypeptide is a member of the leucine-rich repeat protein family and binds at or near the ATP hydrolytic site within the motor domain of the gamma heavy chain from Chlamydomonas outer arm dynein. It consists of an N-terminal helix, a central barrel formed from six leucine-rich repeats that fold as beta beta alpha units, and a C-terminal helical domain that protrudes from the main axis defined by the leucine-rich repeats. Interaction with the gamma heavy chain is likely mediated through a hydrophobic patch on the larger beta sheet face, and the C-terminal region is predicted to insert into the dynein ATP hydrolytic site. Here we have used 1H-15N heteronuclear relaxation measurements obtained at 500 and 600 MHz to refine and validate the LC1 solution structure. In this refined structure, the C-terminal helix is significantly reoriented by more than 20 degrees as compared to the control and provides a more precise understanding of the potential regulatory role of this domain. We also employed the refined structure to perform a dynamic analysis of LC1 using the 600 MHz data set. These results, which were cross validated using the 500 MHz data set, strongly support identification of the predicted LC1 binding surfaces and provide additional insight into the interaction mechanisms of leucine-rich repeat proteins.
Organizational Affiliation:
Department of Biochemistry, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, Connecticut 06030-3305, USA.