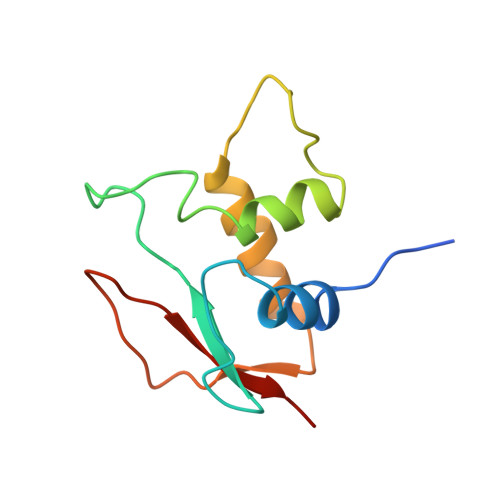
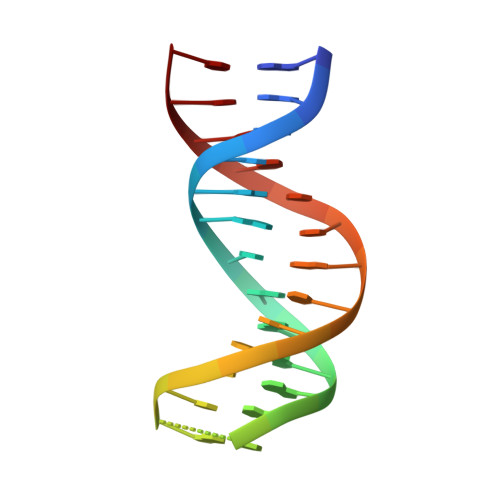
Structure of IRF-1 with bound DNA reveals determinants of interferon regulation.
Escalante, C.R., Yie, J., Thanos, D., Aggarwal, A.K.(1998) Nature 391: 103-106
- PubMed: 9422515
- DOI: https://doi.org/10.1038/34224
- Primary Citation of Related Structures:
1IF1 - PubMed Abstract:
The family of interferon regulatory factor (IRF) transcription factors is important in the regulation of interferons in response to infection by virus and in the regulation of interferon-inducible genes. The IRF family is characterized by a unique 'tryptophan cluster' DNA-binding region. Here we report the crystal structure of the IRF-1 region bound to the natural positive regulatory domain I (PRD I) DNA element from the interferon-beta promoter. The structure provides the first three-dimensional view of a member of the growing IRF family, revealing a new helix-turn-helix motif that latches onto DNA through three of the five conserved tryptophans. The motif selects a short GAAA core sequence through an obliquely angled recognition helix, with an accompanying bending of the DNA axis in the direction of the protein. Together, these features suggest a basis for the occurrence of GAAA repeats within IRF response elements and provide clues to the assembly of the higher-order interferon-beta enhancesome.
Organizational Affiliation:
Structural Biology Program, Department of Physiology and Biophysics, Mount Sinai School of Medicine, New York, New York 10029, USA.