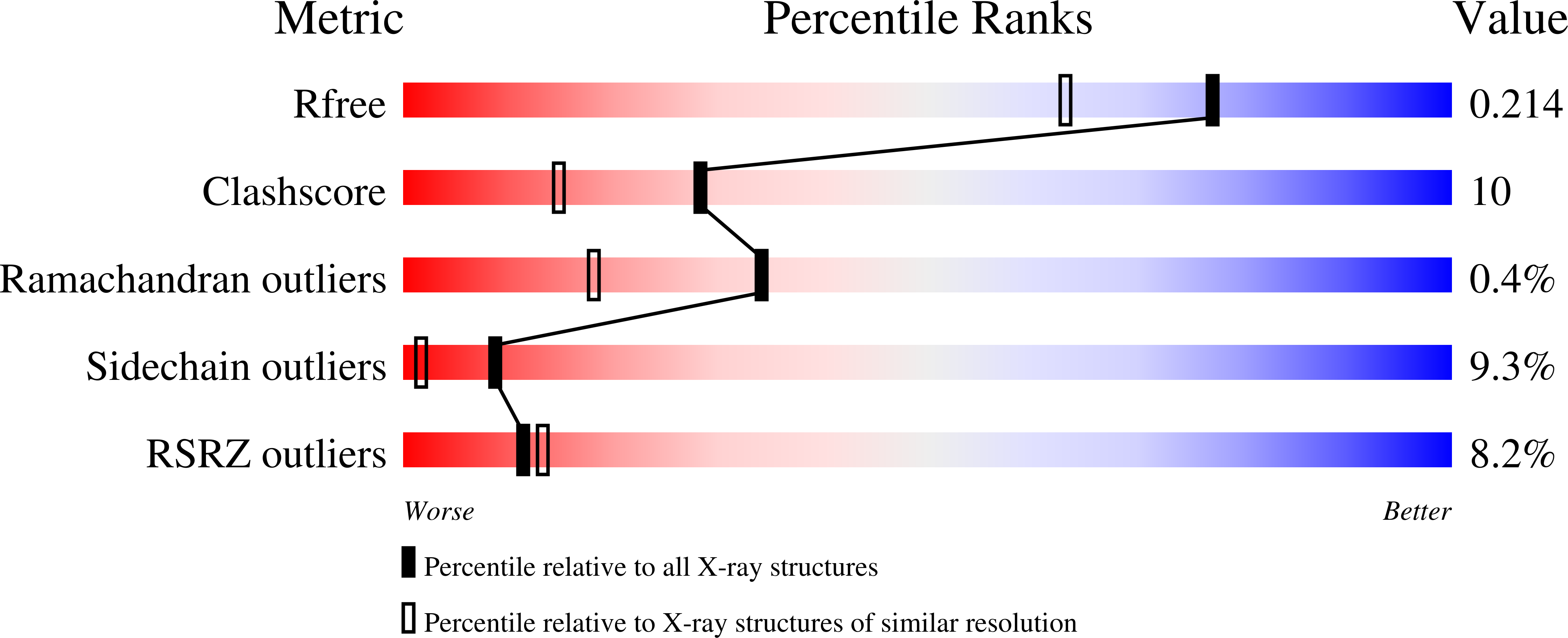
Crystal structures of the Bacillus licheniformis BS3 class A beta-lactamase and of the acyl-enzyme adduct formed with cefoxitin
Fonze, E., Vanhove, M., Dive, G., Sauvage, E., Frere, J.M., Charlier, P.(2002) Biochemistry 41: 1877-1885
- PubMed: 11827533
- DOI: https://doi.org/10.1021/bi015789k
- Primary Citation of Related Structures:
1I2S, 1I2W - PubMed Abstract:
The Bacillus licheniformis BS3 beta-lactamase catalyzes the hydrolysis of the beta-lactam ring of penicillins, cephalosporins, and related compounds. The production of beta-lactamases is the most common and thoroughly studied cause of antibiotic resistance. Although they escape the hydrolytic activity of the prototypical Staphylococcus aureus beta-lactamase, many cephems are good substrates for a large number of beta-lactamases. However, the introduction of a 7alpha-methoxy substituent, as in cefoxitin, extends their antibacterial spectrum to many cephalosporin-resistant Gram-negative bacteria. The 7alpha-methoxy group selectively reduces the hydrolytic action of many beta-lactamases without having a significant effect on the affinity for the target enzymes, the membrane penicillin-binding proteins. We report here the crystallographic structures of the BS3 enzyme and its acyl-enzyme adduct with cefoxitin at 1.7 A resolution. The comparison of the two structures reveals a covalent acyl-enzyme adduct with perturbed active site geometry, involving a different conformation of the omega-loop that bears the essential catalytic Glu166 residue. This deformation is induced by the cefoxitin side chain whose position is constrained by the presence of the alpha-methoxy group. The hydrolytic water molecule is also removed from the active site by the 7beta-carbonyl of the acyl intermediate. In light of the interactions and steric hindrances in the active site of the structure of the BS3-cefoxitin acyl-enzyme adduct, the crucial role of the conserved Asn132 residue is confirmed and a better understanding of the kinetic results emerges.
Organizational Affiliation:
Centre d'Ingénierie des Protéines, Institut de Physique B5 and Institut de Chimie B6, Université de Liège, B-4000 Sart Tilman, Belgium.