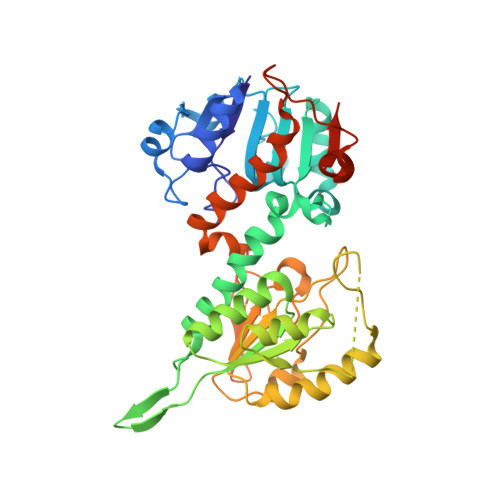
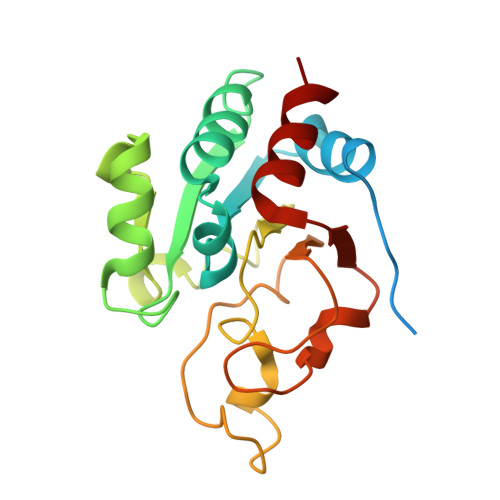
The crystal structure of an asymmetric complex of the two nucleotide binding components of proton-translocating transhydrogenase.
Cotton, N.P., White, S.A., Peake, S.J., McSweeney, S., Jackson, J.B.(2001) Structure 9: 165-176
- PubMed: 11250201
- DOI: https://doi.org/10.1016/s0969-2126(01)00571-8
- Primary Citation of Related Structures:
1HZZ - PubMed Abstract:
Membrane-bound ion translocators have important functions in biology, but their mechanisms of action are often poorly understood. Transhydrogenase, found in animal mitochondria and bacteria, links the redox reaction between NAD(H) and NADP(H) to proton translocation across a membrane. Linkage is achieved through changes in protein conformation at the nucleotide binding sites. The redox reaction takes place between two protein components located on the membrane surface: dI, which binds NAD(H), and dIII, which binds NADP(H). A third component, dII, provides a proton channel through the membrane. Intact membrane-located transhydrogenase is probably a dimer (two copies each of dI, dII, and dIII).
Organizational Affiliation:
School of Biosciences, University of Birmingham, Edgbaston, B15 2TT, Birmingham, United Kingdom.