A helical turn motif in Mss4 is a critical determinant of Rab binding and nucleotide release.
Zhu, Z., Dumas, J.J., Lietzke, S.E., Lambright, D.G.(2001) Biochemistry 40: 3027-3036
- PubMed: 11258916
- DOI: https://doi.org/10.1021/bi002680o
- Primary Citation of Related Structures:
1HXR - PubMed Abstract:
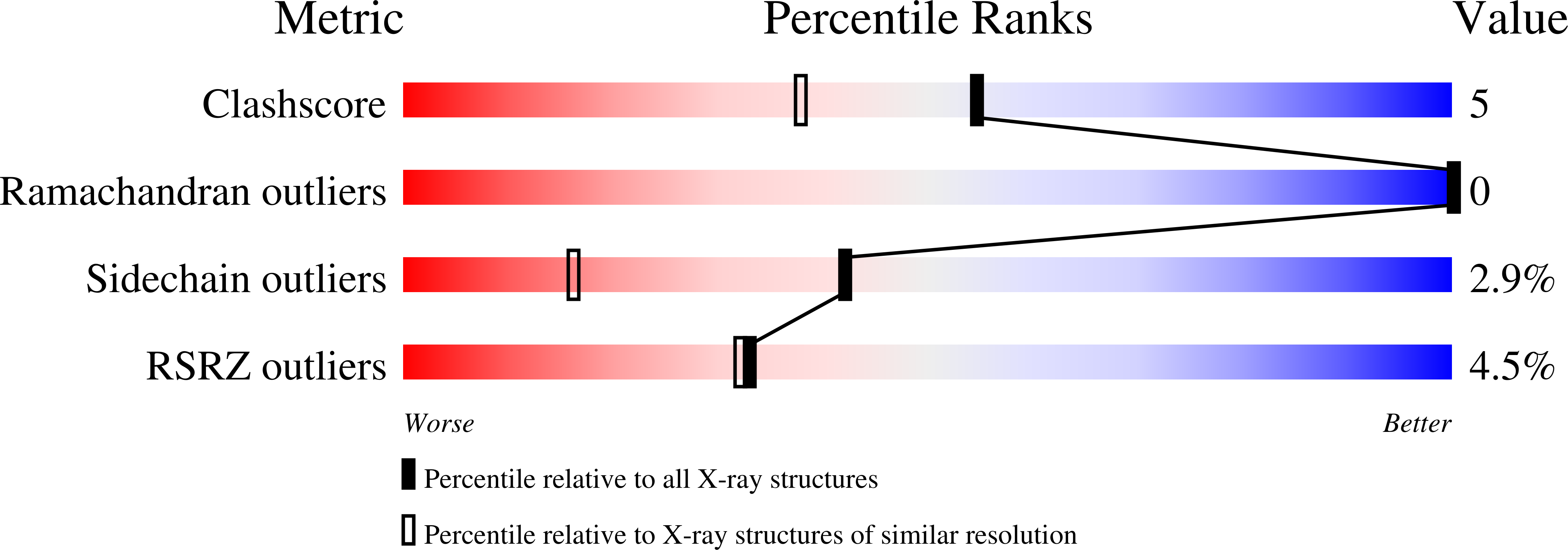
Monomeric Rab GTPases function as ubiquitous regulators of intracellular membrane trafficking. Mss4, an evolutionarily conserved Rab accessory factor, promotes nucleotide release from exocytic but not endocytic Rab GTPases. Here we describe the results of a high-resolution crystallographic and mutational analysis of Mss4. The 1.65 A crystal structure of Mss4 reveals a network of direct and water-mediated interactions that stabilize a partially exposed structural subdomain derived from four highly conserved but nonconsecutive sequence elements. The conserved subdomain contains the invariant cysteine residues required for Zn2+ binding as well as the residues implicated in the interaction with Rab GTPases. A strictly conserved DPhiPhi motif, consisting of an invariant aspartic acid residue (Asp 73) followed by two bulky hydrophobic residues (Met 74 and Phe 75), encodes a prominently exposed 3(10) helical turn in which the backbone is well-ordered but the side chains of the conserved residues are highly exposed and do not engage in intramolecular interactions. Substitution of any of these residues with alanine dramatically impairs nucleotide release activity toward Rab3A, indicating that the DPhiPhi motif is a critical element of the Rab interaction epitope. In particular, mutation of Phe 75 results in a defect as severe as that observed for mutation of Asp 96, which is located near the zinc binding site at the opposite end of the conserved subdomain. Despite severe defects, however, none of the mutant proteins is catalytically dead. Taken together, the results suggest a concerted mechanism in which distal elements of the conserved Rab interaction epitope cooperatively facilitate nucleotide release.
Organizational Affiliation:
Program in Molecular Medicine and Department of Biochemistry & Molecular Pharmacology, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA.