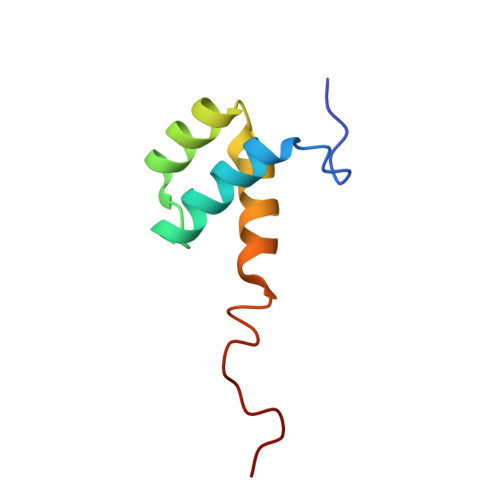
Determination of the three-dimensional structure of the Antennapedia homeodomain from Drosophila in solution by 1H nuclear magnetic resonance spectroscopy.
Billeter, M., Qian, Y., Otting, G., Muller, M., Gehring, W.J., Wuthrich, K.(1990) J Mol Biol 214: 183-197
- PubMed: 2164583
- DOI: https://doi.org/10.1016/0022-2836(90)90155-f
- Primary Citation of Related Structures:
1HOM - PubMed Abstract:
The determination of the three-dimensional structure of the Antennapedia homeodomain from Drosophila in solution is described. The techniques used are 1H nuclear magnetic resonance spectroscopy for the data collection, and calculation of the protein structure with the program DISMAN followed by restrained energy minimization with a modified version of the program AMBER. A group of 19 conformers characterizes a well-defined structure for residues 7 to 59, with an average root-mean-square distance from the backbone atoms of 0.6 A relative to the mean of the 19 structures. The structure contains a helix from residues 10 to 21, a helix-turn-helix motif from residues 28 to 52, which is similar to those reported for several prokaryotic repressor proteins, and a somewhat flexible fourth helix from residues 53 to 59, which essentially forms an extension of the presumed recognition helix, residues 42 to 52. The helices enclose a structurally well-defined molecular core of hydrophobic amino acid side-chains.
Organizational Affiliation:
Institut für Molekularbiologie und Biophysik, Eidgenössische Technische Hochschule-Hönggerberg, Zürich, Switzerland.