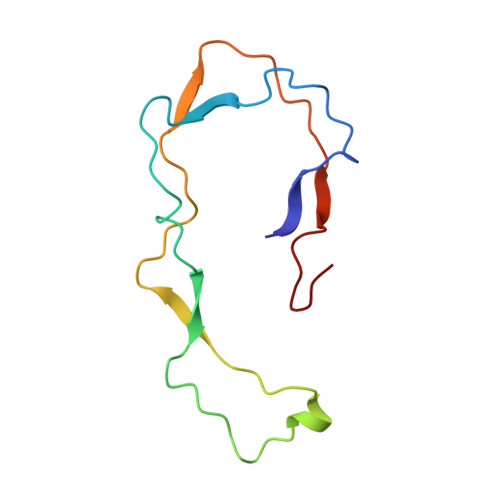
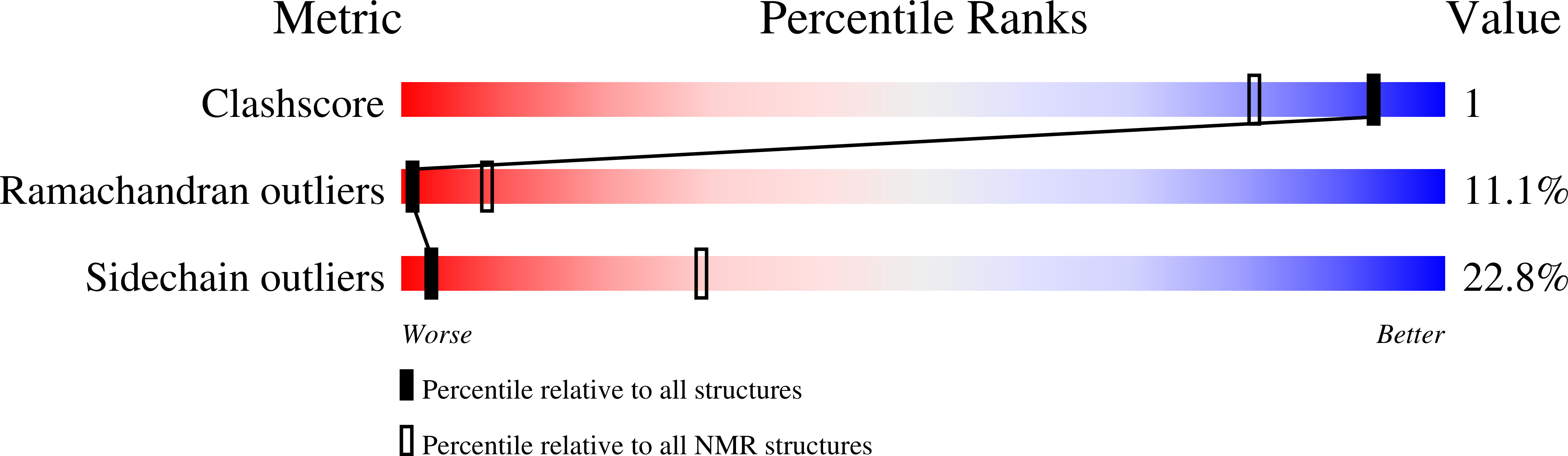NMR Structure of the Calreticulin P-Domain
Ellgaard, L., Riek, R., Herrmann, T., Braun, D., Guntert, P., Helenius, A., Wuthrich, K.(2001) Proc Natl Acad Sci U S A 98: 3133
- PubMed: 11248044
- DOI: https://doi.org/10.1073/pnas.051630098
- Primary Citation of Related Structures:
1HHN - PubMed Abstract:
The NMR structure of the rat calreticulin P-domain, comprising residues 189-288, CRT(189-288), shows a hairpin fold that involves the entire polypeptide chain, has the two chain ends in close spatial proximity, and does not fold back on itself. This globally extended structure is stabilized by three antiparallel beta-sheets, with the beta-strands comprising the residues 189-192 and 276-279, 206-209 and 262-265, and 223-226 and 248-251, respectively. The hairpin loop of residues 227-247 and the two connecting regions between the beta-sheets contain a hydrophobic cluster, where each of the three clusters includes two highly conserved tryptophyl residues, one from each strand of the hairpin. The three beta-sheets and the three hydrophobic clusters form a repeating pattern of interactions across the hairpin that reflects the periodicity of the amino acid sequence, which consists of three 17-residue repeats followed by three 14-residue repeats. Within the global hairpin fold there are two well-ordered subdomains comprising the residues 219-258, and 189-209 and 262-284, respectively. These are separated by a poorly ordered linker region, so that the relative orientation of the two subdomains cannot be precisely described. The structure type observed for CRT(189-288) provides an additional basis for functional studies of the abundant endoplasmic reticulum chaperone calreticulin.
Organizational Affiliation:
Institut für Biochemie, Eidgenössische Technische Hochschule, Universitätstrasse 16, CH-8092 Zurich, Switzerland.