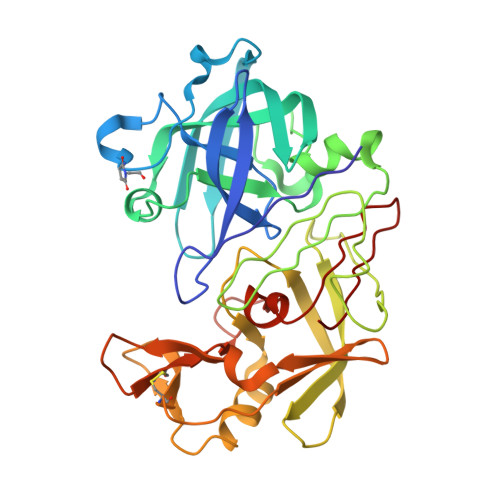
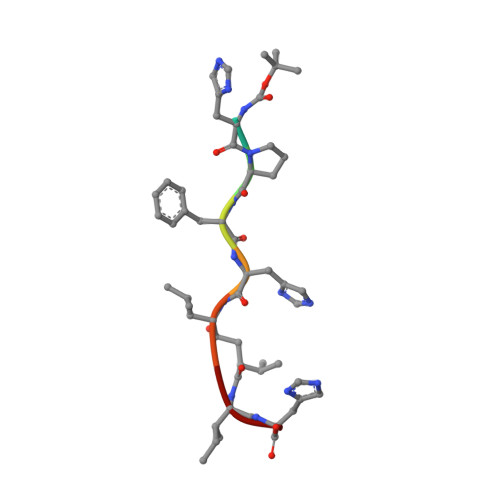
A Neutron Laue Diffraction Study of Endothiapepsin: Implications for the Aspartic Proteinase Mechanism
Coates, L., Erskine, P.T., Wood, S.P., Myles, D.A.A., Cooper, J.B.(2001) Biochemistry 40: 13149
- PubMed: 11683623
- DOI: https://doi.org/10.1021/bi010626h
- Primary Citation of Related Structures:
1GKT - PubMed Abstract:
Current proposals for the catalytic mechanism of aspartic proteinases are largely based on X-ray structures of bound oligopeptide inhibitors possessing nonhydrolyzable analogues of the scissile peptide bond. However, the positions of protons on the catalytic aspartates and the ligand in these complexes have not been determined with certainty. Thus, our objective was to locate crucial protons at the active site of an inhibitor complex since this will have major implications for a detailed understanding of the mechanism of action. We have demonstrated that high-resolution neutron diffraction data can be collected from crystals of the fungal aspartic proteinase endothiapepsin bound to a transition state analogue (H261). The neutron structure of the complex has been refined at a resolution of 2.1 A to an R-factor of 23.5% and an R(free) of 27.4%. This work represents the largest protein structure studied to date by neutron crystallography at high resolution. The neutron data demonstrate that 49% of the main chain nitrogens have exchanged their hydrogen atoms with D2O in the mother liquor. The majority of residues resisting exchange are buried within core beta-sheet regions of the molecule. The neutron maps confirm that the protein has a number of buried ionized carboxylate groups which are likely to give the molecule a net negative charge even at very low pH, thereby accounting for its low pI. The functional groups at the catalytic center have clearly undergone H-D exchange despite being buried by the inhibitor occupying the active site cleft. Most importantly, the data provide convincing evidence that Asp 215 is protonated and that Asp 32 is the negatively charged residue in the transition state complex. This has an important bearing on mechanistic proposals for this class of proteinase.
Organizational Affiliation:
Division of Biochemistry and Molecular Biology, School of Biological Sciences, University of Southampton, Southampton SO16 7PX, UK.