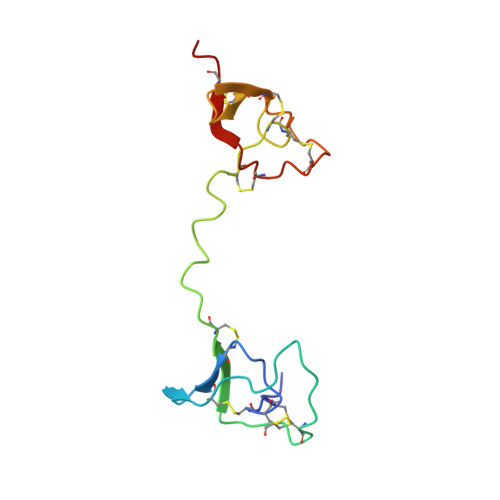
The solution structure of C1-T1, a two-domain proteinase inhibitor derived from a circular precursor protein from Nicotiana alata.
Schirra, H.J., Scanlon, M.J., Lee, M.C., Anderson, M.A., Craik, D.J.(2001) J Mol Biol 306: 69-79
- PubMed: 11178894
- DOI: https://doi.org/10.1006/jmbi.2000.4318
- Primary Citation of Related Structures:
1FYB - PubMed Abstract:
A two-domain portion of the proteinase inhibitor precursor from Nicotiana alata (NaProPI) has been expressed and its structure determined by NMR spectroscopy. NaProPI contains six almost identical 53 amino acid repeats that fold into six highly similar domains; however, the sequence repeats do not coincide with the structural domains. Five of the structural domains comprise the C-terminal portion of one repeat and the N-terminal portion of the next. The sixth domain contains the C-terminal portion of the sixth repeat and the N-terminal portion of the first repeat. Disulphide bonds link these C and N-terminal fragments to generate the clasped-bracelet fold of NaProPI. The three-dimensional structure of NaProPI is not known, but it is conceivable that adjacent domains in NaProPI interact to generate the circular "bracelet" with the N and C termini in close enough proximity to facilitate formation of the disulphide bonds that form the "clasp". The expressed protein, examined in the current study, comprises residues 25-135 of NaProPI and encompasses the first two contiguous structural domains, namely the chymotrypsin inhibitor C1 and the trypsin inhibitor T1, joined by a five-residue linker, and is referred to as C1-T1. The tertiary structure of each domain in C1-T1 is identical to that found in the isolated inhibitors. However, no nuclear Overhauser effect contacts are observed between the two domains and the five-residue linker adopts an extended conformation. The absence of interactions between the domains indicates that adjacent domains do not specifically interact to drive the circularisation of NaProPI. These results are in agreement with recent data which describe similar PI precursors from other members of the Solanaceae having two, three, or four repeats. The lack of strong interdomain association is likely to be important for the function of individual inhibitors by ensuring that there is no masking of reactive sites upon release from the precursor.
Organizational Affiliation:
Institute for Molecular Bioscience, University of Queensland, St. Lucia, Queensland, 4072, Australia.