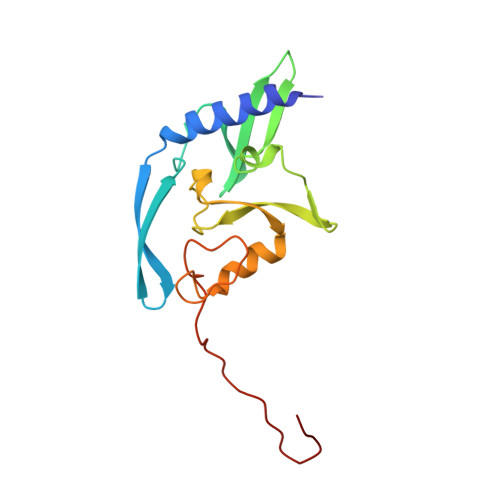
Conformational changes and cleavage by the homing endonuclease I-PpoI: a critical role for a leucine residue in the active site.
Galburt, E.A., Chadsey, M.S., Jurica, M.S., Chevalier, B.S., Erho, D., Tang, W., Monnat Jr., R.J., Stoddard, B.L.(2000) J Mol Biol 300: 877-887
- PubMed: 10891275
- DOI: https://doi.org/10.1006/jmbi.2000.3874
- Primary Citation of Related Structures:
1EVW, 1EVX - PubMed Abstract:
The homing endonuclease I-PpoI severely bends its DNA target, resulting in significant deformations of the minor and major groove near the scissile phosphate groups. To study the role of conformational changes within the protein catalyst and the DNA substrate, we have determined the structure of the enzyme in the absence of bound DNA, performed gel retardation analyses of DNA binding and bending, and have mutagenized a leucine residue that contacts an adenine nucleotide at the site of cleavage. The structure of the L116A/DNA complex has been determined and the effects of the mutation on affinity and catalysis have been measured. The wild-type protein displays a rigid-body rotation of its individual subunits upon DNA binding. Homing site DNA is not detectably bent in the absence of protein, but is sharply bent in both the wild-type and L116A complexes. These results indicate that binding involves a large distortion of the DNA and a smaller change in protein conformation. Leucine 116 is critical for binding and catalysis: it appears to be important for forming a well-ordered protein-DNA complex at the cleavage site, for maximal deformation of the DNA, and for desolvation of the nucleotide bases that are partially unstacked in the enzyme complex.
Organizational Affiliation:
Fred Hutchinson Cancer Research Center and the Graduate Programs in Molecular and Cell Biology and Biomolecular Structure and Design, 1100 Fairview Ave. N. A3-023, Seattle, WA, 98109, USA.