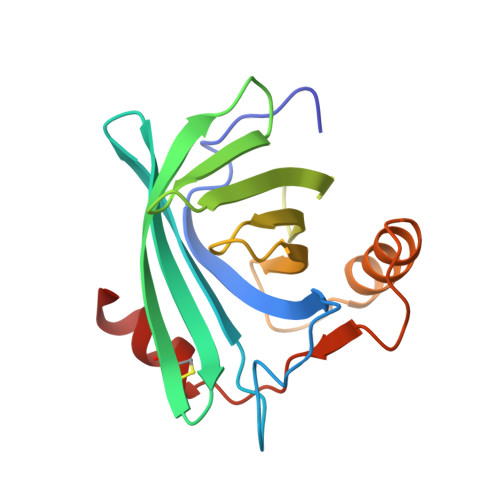
Structure of the epididymal retinoic acid binding protein at 2.1 A resolution.
Newcomer, M.E.(1993) Structure 1: 7-18
- PubMed: 8069623
- DOI: https://doi.org/10.1016/0969-2126(93)90004-z
- Primary Citation of Related Structures:
1EPA, 1EPB - PubMed Abstract:
Androgen-dependent proteins in the lumen of the epididymis are required for sperm maturation. One of these is a retinoic acid binding protein, E-RABP, which binds both all-trans and 9-cis retinoic acid. The other retinoid-binding proteins whose structures are known do not bind 9-cis retinoids.
Organizational Affiliation:
Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-0146.