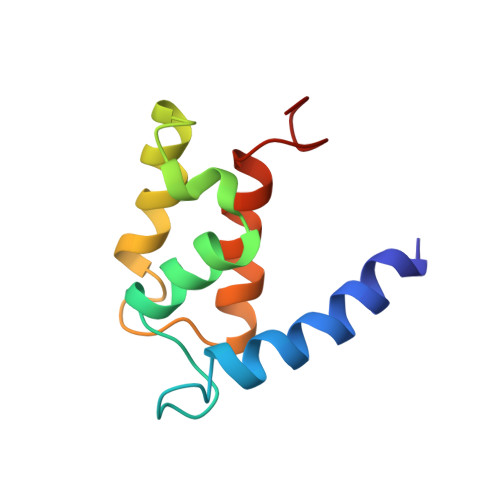
The solution structure of the bovine S100B protein dimer in the calcium-free state.
Kilby, P.M., Van Eldik, L.J., Roberts, G.C.(1996) Structure 4: 1041-1052
- PubMed: 8805590
- DOI: https://doi.org/10.1016/s0969-2126(96)00111-6
- Primary Citation of Related Structures:
1CFP - PubMed Abstract:
S100B (S100beta) is a member of the S100 family of small calcium-binding proteins: members of this family contain two helix-loop-helix calcium-binding motifs and interact with a wide range of proteins involved mainly in the cytoskeleton and cell proliferation. S100B is a neurite-extension factor and levels of S100B are elevated in the brains of patients with Alzheimer's disease or Down's syndrome: the pattern of S100B overexpression in Alzheimer's disease correlates with the pattern of neuritic-plaque formation. Identification of a growing class of S100 proteins and the likely neurochemical importance of S100B make the determination of the structure of S100B of interest.
Organizational Affiliation:
Department of Biochemistry and Biological NMR Centre, Adrian Building, University of Leicester, Leicester, LE1 7RH, UK. pmk@le.ac.uk