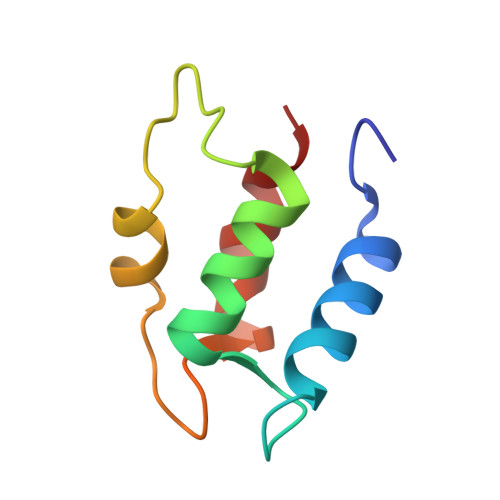
Three-dimensional solution structure of Ca(2+)-loaded porcine calbindin D9k determined by nuclear magnetic resonance spectroscopy.
Akke, M., Drakenberg, T., Chazin, W.J.(1992) Biochemistry 31: 1011-1020
- PubMed: 1734952
- DOI: https://doi.org/10.1021/bi00119a009
- Primary Citation of Related Structures:
1CB1 - PubMed Abstract:
The three-dimensional solution structure of native, intact porcine calbindin D9k has been determined by distance geometry and restrained molecular dynamics calculations using distance and dihedral angle constraints obtained from 1H NMR spectroscopy. The protein has a well-defined global fold consisting of four helices oriented in a pairwise antiparallel manner such that two pairs of helix-loop-helix motifs (EF-hands) are joined by a linker segment. The two EF-hands are further coupled through a short beta-type interaction between the two Ca(2+)-binding loops. Overall, the structure is very similar to that of the highly homologous native, minor A form of bovine calbindin D9k determined by X-ray crystallography [Szebenyi, D. M. E., & Moffat, K. (1986) J. Biol. Chem. 261, 8761-8776]. A model structure built from the bovine calbindin D9k crystal structure shows several deviations larger than 2 A from the experimental distance constraints for the porcine protein. These structural differences are efficiently removed by subjecting the model structure to the experimental distance and dihedral angle constraints in a restrained molecular dynamics protocol, thereby generating a model that is very similar to the refined distance geometry derived structures. The N-terminal residues of the intact protein that are absent in the minor A form appear to be highly flexible and do not influence the structure of other regions of the protein. This result is important because it validates the conclusions drawn from the wide range of studies that have been carried out on minor A forms rather than the intact calbindin D9k.
Organizational Affiliation:
Department of Molecular Biology, Scripps Research Institute, La Jolla, California 92037.