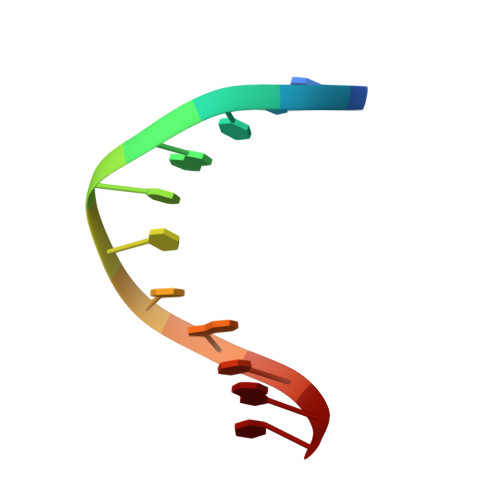
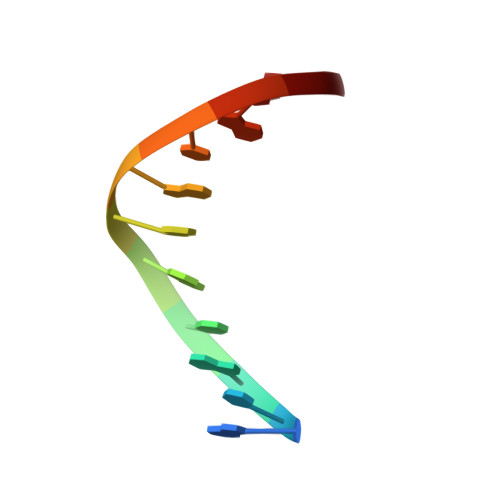
Solution structure of a DNA duplex containing the exocyclic lesion 3,N4-etheno-2'-deoxycytidine opposite 2'-deoxyguanosine.
Cullinan, D., Johnson, F., Grollman, A.P., Eisenberg, M., de los Santos, C.(1997) Biochemistry 36: 11933-11943
- PubMed: 9305987
- DOI: https://doi.org/10.1021/bi9705725
- Primary Citation of Related Structures:
1B6X - PubMed Abstract:
Vinyl chloride reacts with cellular DNA producing 3,N4-etheno-2'-deoxycytidine (epsilonC) along with other exocyclic adducts. The solution structure of an oligodeoxynucleotide duplex containing an epsilonC.dG base pair was determined by high-resolution NMR spectroscopy and molecular dynamics simulations. NMR data indicated that the duplex adopts a right-handed helical structure having all residues in anti orientation around the glycosylic torsion angle. The epsilonC adduct has a sugar pucker in the C3'-endo/C4'-exo region while the rest of the residues are in the C2'-endo/C3'-exo range. NOE interactions established Watson-Crick alignments for canonical base pairs of the duplex. The imino proton of the lesion-containing base pair resonated as a sharp signal that was resistant to water exchange, suggesting hydrogen bonding. Restrained molecular dynamics simulations generated three-dimensional models in excellent agreement with the spectroscopic data. The refined structures are slightly bent at the lesion site without major perturbations of the sugar-phosphate backbone. The adduct is displaced and shifted toward the major groove of the helix while its partner on the complementary strand remains stacked. The epsilonC(anti).dG(anti) base pair alignment is sheared and stabilized by the formation of hydrogen bonds. The biological implications of structures of epsilonC-containing DNA duplexes are discussed.
Organizational Affiliation:
Department of Pharmacological Sciences, State University of New York at Stony Brook, Stony Brook, New York 11794-8651, USA.