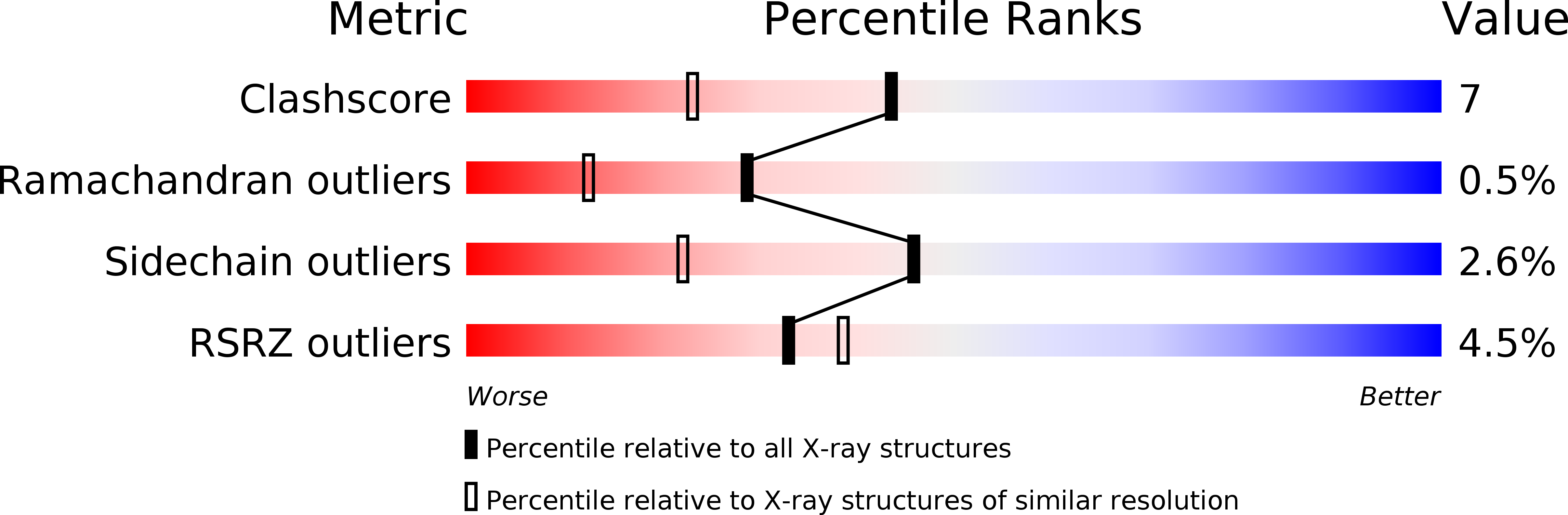
X-ray structure of pyrrolidone carboxyl peptidase from the hyperthermophilic archaeon Thermococcus litoralis.
Singleton, M., Isupov, M., Littlechild, J.(1999) Structure 7: 237-244
- PubMed: 10368293
- DOI: https://doi.org/10.1016/s0969-2126(99)80034-3
- Primary Citation of Related Structures:
1A2Z - PubMed Abstract:
Pyrrolidone carboxyl peptidases (pcps) are a group of exopeptidases responsible for the hydrolysis of N-terminal pyroglutamate residues from peptides and proteins. The bacterial and archaeal pcps are members of a conserved family of cysteine proteases. The pcp from the hyperthermophilic archaeon Thermococcus litoralis is more thermostable than the bacterial enzymes with which it has up to 40% sequence identity. The pcp activity in archaea and eubacteria is proposed to be involved in detoxification processes and in nutrient metabolism; eukaryotic counterparts of the enzyme are involved in the processing of biologically active peptides.
Organizational Affiliation:
Schools of Chemistry and Biological Sciences University of Exeter Stocker Road Exeter EX4 4QD, UK.